White Paper by Wei Adelyn Tsai
Reviewed by: Greg Hollenbeck, Thia Hanania.
Dehydroepiandrosterone (DHEA), or Prasterone, was first found from urine in 1934 by Adolf Butenandt and Hans Dannenbaum. Its sulfated metabolite, dehydroepiandrosterone sulfate (DHEA-S) was isolated from urine ten years after. In the 1960s, the scientific community started assessing the functions of DHEA and DHEA-S in the brain (Pope et al., 2003). Today, it is known that DHEA and DHEA-S are neurosteroid hormones produced via the steroid biosynthetic pathways (Fig.1) (Baulieu & Robel, 1998). Both DHEA and DHEA-S exert a wide array of neurobiological actions that lead to neuroprotection, neurogenesis, and neuronal survival (Fig.2) (Maninger et al., 2010). Importantly, DHEA can cross the blood-brain barrier (Stárka et al., 2015). Therefore, they have been reported in both preclinical and clinical studies to play roles in neuropsychiatric and neurological diseases. This paper will specifically focus on the potential therapeutic effects of the commercially available supplement DHEA in depression, schizophrenia, and dementia. The biological actions of Its sulfated form, DHEA-S in these three conditions will also be discussed.
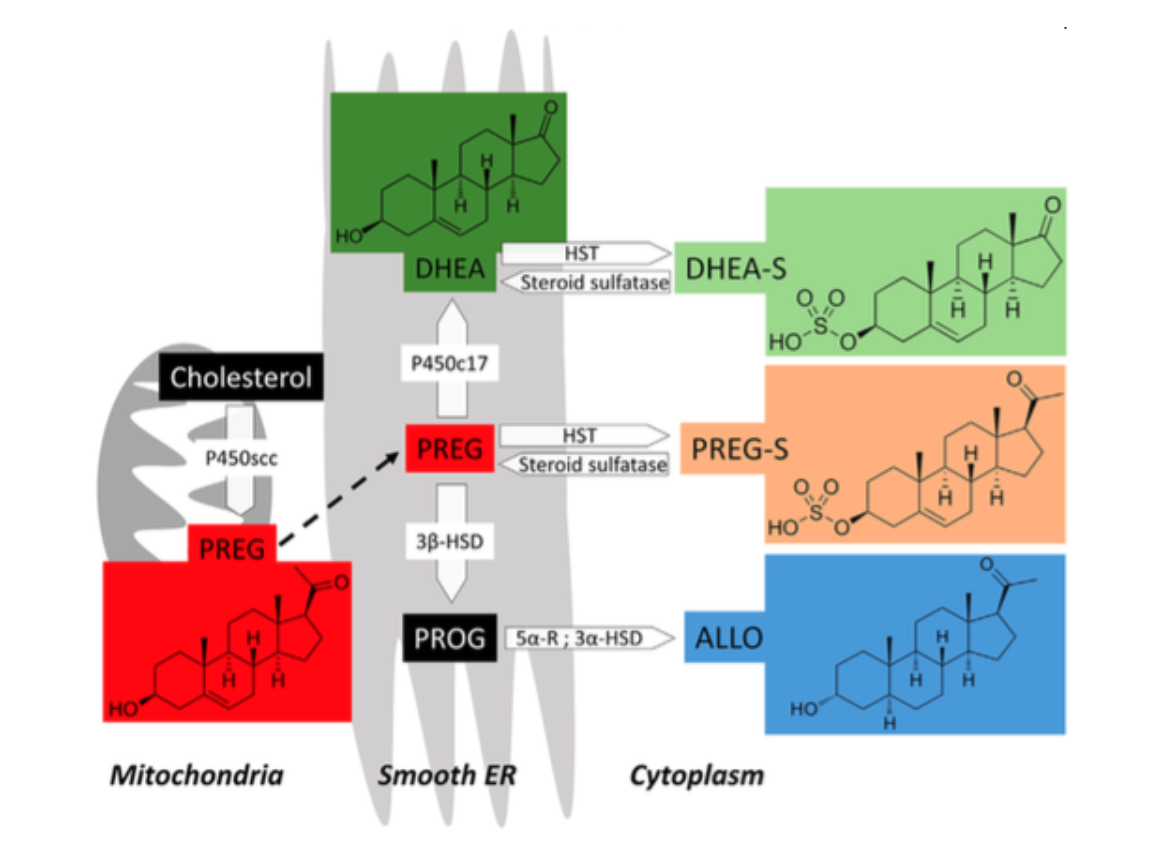
Fig.1 Steroid biosynthesis of DHEA, and DHEA-S. Cholesterol is transformed into pregnenolone (PREG) in the mitochondria by the cytochrome P450 side-chain cleavage (P450scc). PREG is transported to the smooth endoplasmic reticulum (ER) and metabolized into DHEA by cytochrome P450 17α- hydroxylase/C17,20-lyase (P450c17). DHEA-S is produced from the sulfation of DHEA via hydroxysteroid sulfotransferase (HST) in the cytoplasm, and desulfated by a steroid sulfatase; other neurosteroids involved in this pathway are Progesterone (PROG), sulfated PREG (PREG-S), and allopregnanolone (ALLO). For a detailed description of the other molecules please direct to Pregnenolone (Schverer et al., 2018).
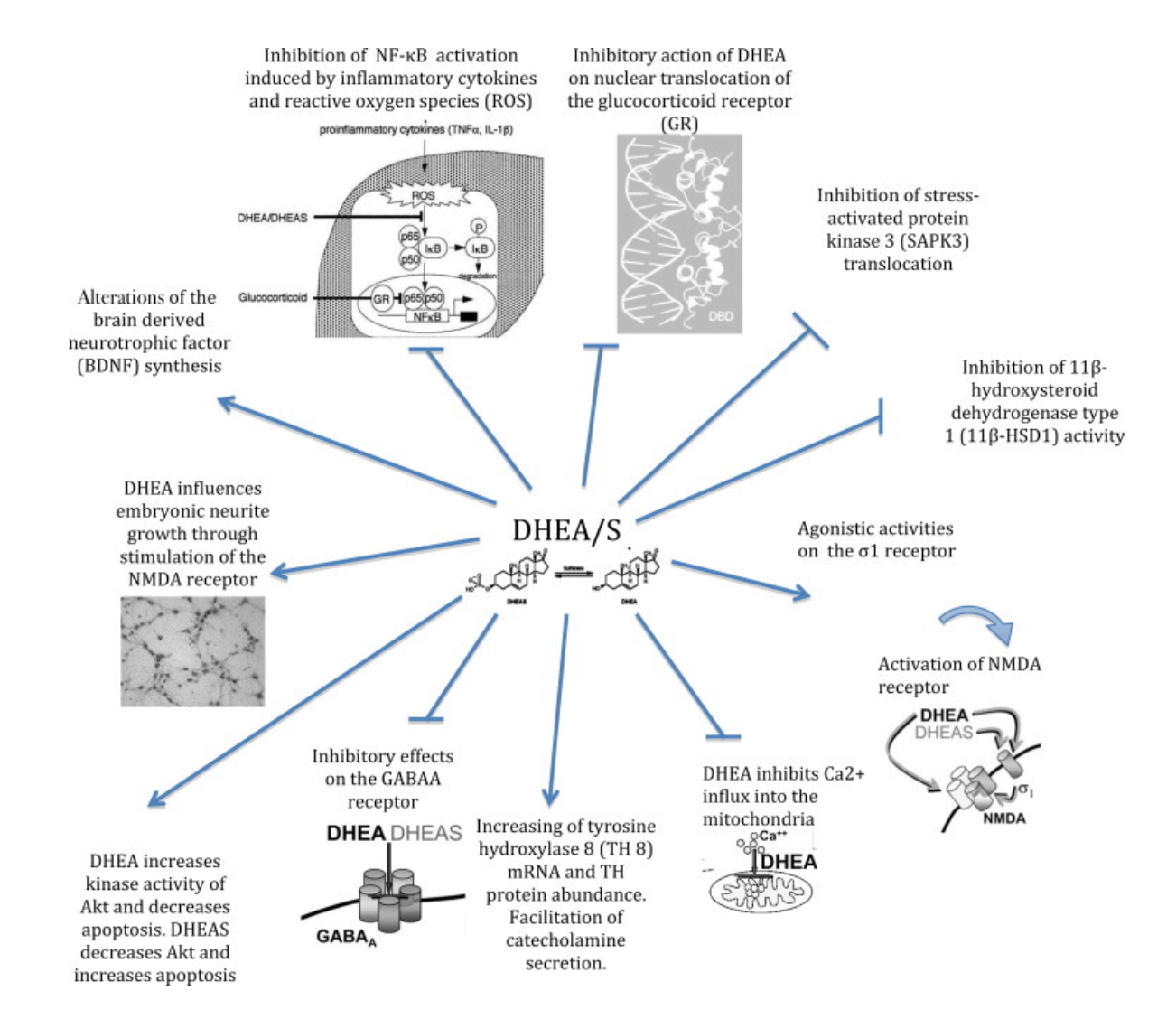
Fig.2 Biological mechanisms of DHEA and DHEA-S in the brain (Maggio et al., 2015).
1) Depression:
Chronic depression is characterized by compromised N-methyl-D-aspartate (NMDA) receptor function and glutamatergic signaling (Fishback et al., 2010). Importantly, DHEA and DHEA-S are agonists of NMDA receptors through direct binding or mediated by binding to σ1 receptors (Compagnone & Mellon, 1998), suggesting the possible anti-depressive effects of DHEA and DHEA-S. DHEA and DHEA-S also promote catecholamine synthesis and secretion. Catecholamines include dopamine (DA) and norepinephrine (NE). Catecholamines regulate emotion and cognition in the brain (Kobayashi, 2001), and previous research suggested that both DA and NE have antidepressant effects (Briley & Chantal, 2011; Dunlop & Nemeroff, 2007). Therefore, by stimulating the secretion and synthesis of catecholamines (Pérez-Neri et al., 2008; Charalampopoulos et al., 2005), DHEA and DHEA-S can be a potential useful antidepressant.
Moreover, depression is accompanied by increased inflammation (Kubera et al., 2011) and oxidative stress in the brain (Maes et al., 2011). Inflammatory cytokines also activate nuclear factor-κB (NF-κB), a transcription factor inducing expression of genes involved in inflammation, cell proliferation, and cell survival (Aragno et al., 2002), in depressive patients (Miklowitz et al., 2016). Decreased pro-inflammatory cytokines by DHEA and DHEA-S have been observed in rats fed with DHEA (Kimura et al., 1998), in rat brain tissues culturing with DHEA (Kipper‐Galperin et al., 1999), and in human blood cells (Straub et al., 1998) incubated with DHEA. DHEA was also able to balance the oxidative state and suppress the activation of NF-κB in the hippocampus of diabetic rats (Aragno et al., 2002). These suggest that DHEA and DHEA-S can potentially mitigate depression by acting as anti-inflammatory and antioxidant agents and the inhibitors of NF-κB activation.
Depression has been associated with raised levels of cortisol, a type of glucocorticoid (GC) hormones (Steckler et al., 1999). DHEA has anti-glucocorticoid effects, possibly by inhibiting GC receptor translocation into the nucleus, or by inhibiting the expression or activity of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1), an enzyme catalyzing the conversion of cortisone (an inactive glucocorticoid) to cortisol (an active glucocorticoid). Another possible mechanism is reducing the nuclear translocation of stress-activated protein kinase 3 (SAPK3) induced by corticosterone, which may prevent neurotoxic actions of corticosterone (Maninger et al., 2010). In humans, lower salivary DHEA to cortisol ratio was observed in major depression in adults (Michael et al., 2000). Therefore, DHEA may be a treatment for depression by reducing the cortisol level.
The biological functions of DHEA and DHEA-S described above make them possible antidepressants. Attempts to use DHEA and/or DHEA-S levels in the plasma or serum as markers of depression have revealed inconclusive results. On the other hand, it may be more accurate to measure the cortisol/DHEA ratio, with a higher ratio being found in depression (Markopoulou et al., 2009). Clinical studies have been done to assess the efficacy of DHEA and DHEA-S as antidepressants. In a double-blind, placebo-controlled trial, 22 depressed patients received either DHEA (maximum dose = 90mg/d) or a placebo for 6 weeks. Some patients were medication-free while others maintained antidepressant medications. 5 out of 11 patients treated with DHEA showed a 50% greater improvement in depression rating while no placebo-treated patients showed this level of improvement (Wolkowitz et al., 1999). This suggested DHEA may be used adjunctively to other antidepressants. Another 12 weeks double-blind, randomized, placebo-controlled, crossover treatment study in 23 men and 23 women with midlife-onset depression found that DHEA (maximum dose= 450 mg/d) enhanced mood (Schmidt et al., 2005). Recently, a meta-analysis of 22 studies showed DHEA to be promising for treating depression in old and young patients, especially in depression that is mild or resistant to conventional therapy (Peixoto et al., 2014). Therefore, supplementation with DHEA may be helpful for depressive symptoms.
2) Schizophrenia
In a recent meta-analysis, it was found that DHEA-S, but not DHEA is elevated in patients with first-episode schizophrenia. However, the elevation of DHEA-S is blunted as the disease progresses (Misiak et al., 2018). Since DHEA is rapidly metabolized into DHEA-S, changes in DHEA-S may reflect recent changes in the DHEA level (Beyazyüz et al., 2014). It has been proposed that early alterations of neurosteroids are a protective response to neurobiological stress and can reduce adverse clinical features (Beyazyüz et al., 2014; Strous et al., 2004). Notably, in medicated schizophrenic patients, several groups reported elevated serum DHEA levels (Gallagher et al., 2007; Harris et al., 2001; Ritsner et al., 2006).
Changes of DHEA and DHEA-S in different stages of schizophrenia and in medicated patients suggest their potential therapeutic effects. It is possible that, as agonists of NMDA receptors (Compagnone & Mellon, 1998), DHEA or DHEA-S therapy might be therapeutic for NMDA receptors hypofunction implicated in schizophrenia (Balu, 2016). Hypoactive mesocortical DA projections is another pathology of schizophrenia (Abi-Dargham & Moore, 2003), so DHEA and DHEA-S stimulation of DA secretion and production in the frontal cortex may be another therapeutic mechanism (Shulman & Tibbo, 2005).
Indeed, clinical studies have shown the therapeutic effects of DHEA administered on schizophrenia patients. Strous et al. found increased DHEA and DHEA-S plasma level and improvement in negative, depressive, and anxiety symptoms in schizophrenic patients after 6 weeks of DHEA therapy (maximum dose=100mg/d) (Strous et al., 2003). The same group also found DHEA co-administration (maximum dose=150mg/d) with antipsychotic olanzapine for 12 weeks improved negative symptoms and some improvement in extrapyramidal motor symptoms (EPS), that is, Parkinsonism and akathisia (Strous et al., 2007). Another study also found a significant decrease in Parkinsonism but not akathisia in antipsychotic-medicated patients co-treated with 100mg/d DHEA (Nachshoni et al., 2005).
3) Dementia
σ1 receptors agonists like DHEA and DHEA-S have anti-amnesic properties through enhancing cholinergic and glutamatergic systems (Maurice & Goguadze, 2017; van Waarde et al., 2011). Both cholinergic and glutamatergic systems modulate learning and memory (Gasbarri & Pompili, 2014; Gold, 2003). DHEA-S, by stimulating σ1 receptors, also induced long-term potentiation, a process critical for learning and memory, in the rat hippocampus, a brain region involved in cognitive processes (Chen et al., 2006). Furthermore, the antioxidative effects of DHEA produced protective effects in rat hippocampal cells and human hippocampal tissues from AD patients (Bastianetto et al., 1999). DHEA also rescued the increase of β-site AβPP-cleaving enzyme, an enzyme responsible for the accumulation of amyloid-β (Aβ) in AD, in neurons in response to oxidative stress (Tamagno et al., 2003). Other studies show that DHEA is protective against Aβ protein toxicity, the hallmark of AD, in cell models (Cardounel et al., 1999; Tamagno et al., 2003).
In the serum, DHEA or DHEA-S concentration alone does not clearly associate with dementia, while DHEA-S/cortisol ratio seems to better distinguish demented patients, with lower DHEA-S/cortisol ratio being reported in AD and demented patients. In the cerebral spinal fluid, a lower DHEA-S/DHEA ratio was observed in demented patients, and it was suggested that DHEA-S/DHEA ratio is a better marker of dementia than the concentrations of either steroid alone (Maninger et al., 2010). A few studies have been done to assess DHEA and DHEA-S therapies for dementia. In one open-label study on seven patients with multi-infarct dementia treating with 4 weeks of 200mg/d intravenous DHEA-S, three patients had improved decrease of daily activities and emotional disturbances and two patients had improved electroencephalogram patterns (Azuma et al., 1999). In another study on 33 un-medicated AD patients treated with 6 months of DHEA (100mg/d), DHEA had a transient, tendential effect on improved cognitive performance at month 3, and at month 6, the DHEA group still only showed a trend for improvement but not reached significance (Wolkowitz et al., 2003). Importantly, in a study, Yamada et al showed 6 months of treatment with 25mg/d of DHEA could improve cognitive scores and maintained basic activities of daily living in older women with mild to moderate cognitive impairment (Yamada et al., 2010). Since mild cognitive impairment may result in dementia (Hugo & Ganguli, 2014), this study indicates that DHEA can prevent further cognitive decline to dementia. These three pilot studies provided promising results to treat or prevent dementia and larger clinical trials are still needed.
In conclusion, DHEA and DHEA-S exert many biological actions that produce beneficial effects on brain functions. Based on preclinical studies, it was suggested that the timing of administration, doses of DHEA and DHEA-S, and the presence of extant factors such as the concentrations of other hormones or the physiological states of the organism, could all affect the outcomes produced from DHEA and DHEA-S administration (Maninger et al., 2010). In humans, these factors should all be considered when consuming DHEA. One important factor may be age since DHEA declines as ages (Quinn et al., 2018). The clinical studies discussed here mainly used DHEA, because it is orally available and can cross the blood-brain barrier. DHEA showed a wide range of tolerable dosage, from 25mg/d to 450mg/d. However, the dosage taken should be carefully decided depending on the target symptoms. It is also of note that although DHEA and DHEA-S have been examined and proposed to be potentially therapeutic for depression, schizophrenia, and dementia, so far only the beneficial effects of DHEA in depression have been better elucidated in the clinical studies.
References
Abi-Dargham, A., & Moore, H. (2003). Prefrontal DA Transmission at D1 Receptors and the Pathology of Schizophrenia. The Neuroscientist, 9(5), 404–416. https://doi.org/10.1177/1073858403252674
Aragno, M., Mastrocola, R., Brignardello, E., Catalano, M., Robino, G., Manti, R., Parola, M., Danni, O., & Boccuzzi, G. (2002). Dehydroepiandrosterone Modulates Nuclear Factor-κB Activation in Hippocampus of Diabetic Rats. Endocrinology, 143(9), 3250–3258. https://doi.org/10.1210/en.2002-220182
Azuma, T., Nagai, Y., Saito, T., Funauchi, M., Matsubara, T., & Sakoda, S. (1999). The effect of dehydroepiandrosterone sulfate administration to patients with multi-infarct dementia. Journal of the Neurological Sciences, 162(1), 69–73. https://doi.org/10.1016/S0022-510X(98)00295-0
Balu, D. T. (2016). The NMDA Receptor and Schizophrenia (pp. 351–382). https://doi.org/10.1016/bs.apha.2016.01.006
Bastianetto, S., Ramassamy, C., Poirier, J., & Quirion, R. (1999). Dehydroepiandrosterone (DHEA) protects hippocampal cells from oxidative stress-induced damage. Molecular Brain Research, 66(1–2), 35–41. https://doi.org/10.1016/S0169-328X(99)00002-9
Beyazyüz, M., Albayrak, Y., Beyazyüz, E., Ünsal, C., & Göka, E. (2014). Increased serum dehydroepiandrosterone sulfate in the first episode but not in subsequent episodes in male patients with schizophrenia. Neuropsychiatric Disease and Treatment, 10, 687–693. https://doi.org/10.2147/NDT.S61406
Briley, M., & Chantal, M. (2011). The importance of norepinephrine in depression. Neuropsychiatric Disease and Treatment, 9. https://doi.org/10.2147/NDT.S19619
Cardounel, A., Regelson, W., & Kalimi, M. (1999). Dehydroepiandrosterone Protects Hippocampal Neurons Against Neurotoxin-Induced Cell Death: Mechanism of Action. Proceedings of the Society for Experimental Biology and Medicine, 222(2), 145–149. https://doi.org/10.1046/j.1525-1373.1999.d01-124.x
Charalampopoulos, I., Dermitzaki, Ε., Vardouli, L., Tsatsanis, C., Stournaras, C., Margioris, Α. Ν., & Gravanis, Α. (2005). Dehydroepiandrosterone Sulfate and Allopregnanolone Directly Stimulate Catecholamine Production via Induction of Tyrosine Hydroxylase and Secretion by Affecting Actin Polymerization. Endocrinology, 146(8), 3309–3318. https://doi.org/10.1210/en.2005-0263
Chen, L., Dai, X.-N., & Sokabe, M. (2006). Chronic administration of dehydroepiandrosterone sulfate (DHEAS) primes for facilitated induction of long-term potentiation via sigma 1 (σ1) receptor: Optical imaging study in rat hippocampal slices. Neuropharmacology, 50(3), 380–392. https://doi.org/10.1016/j.neuropharm.2005.10.015
Compagnone, N. A., & Mellon, S. H. (1998). Dehydroepiandrosterone: A potential signalling molecule for neocortical organization during development. Proceedings of the National Academy of Sciences, 95(8), 4678–4683. https://doi.org/10.1073/pnas.95.8.4678
Dunlop, B. W., & Nemeroff, C. B. (2007). The Role of Dopamine in the Pathophysiology of Depression. Archives of General Psychiatry, 64(3), 327. https://doi.org/10.1001/archpsyc.64.3.327
Fishback, J. A., Robson, M. J., Xu, Y.-T., & Matsumoto, R. R. (2010). Sigma receptors: Potential targets for a new class of antidepressant drug. Pharmacology & Therapeutics, 127(3), 271–282. https://doi.org/10.1016/j.pharmthera.2010.04.003
Gallagher, P., Watson, S., Smith, M., Young, A., & Ferrier, I. (2007). Plasma cortisol-dehydroepiandrosterone (DHEA) ratios in schizophrenia and bipolar disorder. Schizophrenia Research, 90(1–3), 258–265. https://doi.org/10.1016/j.schres.2006.11.020
Gasbarri, A., & Pompili, A. (2014). Involvement of Glutamate in Learning and Memory. In Identification of Neural Markers Accompanying Memory (pp. 63–77). Elsevier. https://doi.org/10.1016/B978-0-12-408139-0.00004-3
Gold, P. (2003). Acetylcholine modulation of neural systems involved in learning and memory. Neurobiology of Learning and Memory, 80(3), 194–210. https://doi.org/10.1016/j.nlm.2003.07.003
Harris, D. S., Wolkowitz, O. M., & Reus, V. I. (2001). Movement Disorder, Memory, Psychiatric Symptoms and Serum DHEA Levels in Schizophrenic and Schizoaffective Patients. The World Journal of Biological Psychiatry, 2(2), 99–102. https://doi.org/10.3109/15622970109027500
Hugo, J., & Ganguli, M. (2014). Dementia and Cognitive Impairment. Clinics in Geriatric Medicine, 30(3), 421–442. https://doi.org/10.1016/j.cger.2014.04.001
Kimura, M., Tanaka, S., Yamada, Y., Kiuchi, Y., Yamakawa, T., & Sekihara, H. (1998). Dehydroepiandrosterone Decreases Serum Tumor Necrosis Factor-α and Restores Insulin Sensitivity: Independent Effect from Secondary Weight Reduction in Genetically Obese Zucker Fatty Rats 1. Endocrinology, 139(7), 3249–3253. https://doi.org/10.1210/endo.139.7.6118
Kipper‐Galperin, M., Galilly, R., Danenberg, H. D., & Brenner, T. (1999). Dehydroepiandrosterone selectively inhibits production of tumor necrosis factor α and Interlukin‐6 in astrocytes. International Journal of Developmental Neuroscience, 17(8), 765–775. https://doi.org/10.1016/S0736-5748(99)00067-2
Kobayashi, K. (2001). Role of Catecholamine Signaling in Brain and Nervous System Functions: New Insights from Mouse Molecular Genetic Study. Journal of Investigative Dermatology Symposium Proceedings, 6(1), 115–121. https://doi.org/10.1046/j.0022-202x.2001.00011.x
Kubera, M., Obuchowicz, E., Goehler, L., Brzeszcz, J., & Maes, M. (2011). In animal models, psychosocial stress-induced (neuro)inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 35(3), 744–759. https://doi.org/10.1016/j.pnpbp.2010.08.026
Maes, M., Galecki, P., Chang, Y. S., & Berk, M. (2011). A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 35(3), 676–692. https://doi.org/10.1016/j.pnpbp.2010.05.004
Maggio, M., De Vita, F., Fisichella, A., Colizzi, E., Provenzano, S., Lauretani, F., Luci, M., Ceresini, G., Dall’Aglio, E., Caffarra, P., Valenti, G., & Ceda, G. P. (2015). DHEA and cognitive function in the elderly. The Journal of Steroid Biochemistry and Molecular Biology, 145, 281–292. https://doi.org/10.1016/j.jsbmb.2014.03.014
Maninger, N., Wolkowitz, O. M., Reus, V. I., Epel, E. S., & Synthia, H. (2010). Neurobiological and Neuropsychiatric Effects of DHEA and DHEA-s. NIH Public Access, 30(1), 65–91. https://doi.org/10.1016/j.yfrne.2008.11.002.Neurobiological
Markopoulou, K., Papadopoulos, A., Juruena, M. F., Poon, L., Pariante, C. M., & Cleare, A. J. (2009). The ratio of cortisol/DHEA in treatment resistant depression. Psychoneuroendocrinology, 34(1), 19–26. https://doi.org/10.1016/j.psyneuen.2008.08.004
Maurice, T., & Goguadze, N. (2017). Sigma-1 (σ1) Receptor in Memory and Neurodegenerative Diseases (pp. 81–108). https://doi.org/10.1007/164_2017_15
Michael, A., Jenaway, A., Paykel, E. S., & Herbert, J. (2000). Altered salivary dehydroepiandrosterone levels in major depression in adults. Biological Psychiatry, 48(10), 989–995. https://doi.org/10.1016/S0006-3223(00)00955-0
Miklowitz, D. J., Portnoff, L. C., Armstrong, C. C., Keenan-Miller, D., Breen, E. C., Muscatell, K. A., Eisenberger, N. I., & Irwin, M. R. (2016). Inflammatory cytokines and nuclear factor-kappa B activation in adolescents with bipolar and major depressive disorders. Psychiatry Research, 241, 315–322. https://doi.org/10.1016/j.psychres.2016.04.120
Misiak, B., Frydecka, D., Loska, O., Moustafa, A. A., Samochowiec, J., Kasznia, J., & Stańczykiewicz, B. (2018). Testosterone, DHEA and DHEA-S in patients with schizophrenia: A systematic review and meta-analysis. Psychoneuroendocrinology, 89, 92–102. https://doi.org/10.1016/j.psyneuen.2018.01.007
Nachshoni, T., Ebert, T., Abramovitch, Y., Assaelamir, M., Kotler, M., Maayan, R., Weizman, A., & Strous, R. (2005). Improvement of extrapyramidal symptoms following dehydroepiandrosterone (DHEA) administration in antipsychotic treated schizophrenia patients: A randomized, double-blind placebo controlled trial. Schizophrenia Research, 79(2–3), 251–256. https://doi.org/10.1016/j.schres.2005.07.029
Owen M. Wolkowitz, Victor I. Reus, Audrey Keebler, N. N., & Mirit Friedland, Louann Brizendine, and E. R. (1999). Double-Blind Treatment of Major Depression With Dehydroepiandrosterone. April, 1–4. papers2://publication/uuid/FE75E72A-E2CF-4372-A613-27B65D9B11F5
Peixoto, C., Devicari Cheda, J., Nardi, A., Veras, A., & Cardoso, A. (2014). The Effects of Dehydroepiandrosterone (DHEA) in the Treatment of Depression and Depressive Symptoms in Other Psychiatric and Medical Illnesses: A Systematic Review. Current Drug Targets, 15(9), 901–914. https://doi.org/10.2174/1389450115666140717111116
Pérez-Neri, I., Montes, S., Ojeda-López, C., Ramírez-Bermúdez, J., & Ríos, C. (2008). Modulation of neurotransmitter systems by dehydroepiandrosterone and dehydroepiandrosterone sulfate: Mechanism of action and relevance to psychiatric disorders. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 32(5), 1118–1130. https://doi.org/10.1016/j.pnpbp.2007.12.001
Pope, J. E., Cupp, M. J., & Tracy, T. S. (2003). Dehydroepiandrosterone (DHEA) (Prasterone). In M. J. Cupp & T. S. Tracy (Eds.), Dietary Supplements: Toxicology and Clinical Pharmacology (pp. 123–147). Humana Press. https://doi.org/10.1007/978-1-59259-303-3_8
Quinn, T. A., Robinson, S. R., & Walker, D. (2018). Dehydroepiandrosterone (DHEA) and DHEA Sulfate: Roles in Brain Function and Disease. In Sex Hormones in Neurodegenerative Processes and Diseases. InTech. https://doi.org/10.5772/intechopen.71141
Ritsner, M., Gibel, A., Ram, E., Maayan, R., & Weizman, A. (2006). Alterations in DHEA metabolism in schizophrenia: Two-month case-control study. European Neuropsychopharmacology, 16(2), 137–146. https://doi.org/10.1016/j.euroneuro.2005.07.007
Schmidt, P. J., Daly, R. C., Bloch, M., Smith, M. J., Danaceau, M. A., Simpson St. Clair, L., Murphy, J. H., Haq, N., & Rubinow, D. R. (2005). Dehydroepiandrosterone Monotherapy in Midlife-Onset Major and Minor Depression. Archives of General Psychiatry, 62(2), 154. https://doi.org/10.1001/archpsyc.62.2.154
Schverer, M., Lanfumey, L., Baulieu, E. E., Froger, N., & Villey, I. (2018). Neurosteroids: non-genomic pathways in neuroplasticity and involvement in neurological diseases. Pharmacology and Therapeutics, 191, 190–206. https://doi.org/10.1016/j.pharmthera.2018.06.011
Shulman, Y., & Tibbo, P. G. (2005). Neuroactive Steroids in Schizophrenia. The Canadian Journal of Psychiatry, 50(11), 695–702. https://doi.org/10.1177/070674370505001109
Stárka, L., Dušková, M., & Hill, M. (2015). Dehydroepiandrosterone: A neuroactive steroid. Journal of Steroid Biochemistry and Molecular Biology, 145, 254–260. https://doi.org/10.1016/j.jsbmb.2014.03.008
Steckler, T., Holsboer, F., & Reul, J. M. H. M. (1999). Glucocorticoids and depression. Best Practice & Research Clinical Endocrinology & Metabolism, 13(4), 597–614. https://doi.org/10.1053/beem.1999.0046
Straub, R. H., Konecna, L., Hrach, S., Rothe, G., Kreutz, M., Schölmerich, J., Falk, W., & Lang, B. (1998). Serum Dehydroepiandrosterone (DHEA) and DHEA Sulfate Are Negatively Correlated with Serum Interleukin-6 (IL-6), and DHEA Inhibits IL-6 Secretion from Mononuclear Cells in Man in Vitro : Possible Link between Endocrinosenescence and Immunosenescence. The Journal of Clinical Endocrinology & Metabolism, 83(6), 2012–2017. https://doi.org/10.1210/jcem.83.6.4876
Strous, R. D., Maayan, R., Lapidus, R., Goredetsky, L., Zeldich, E., Kotler, M., & Weizman, A. (2004). Increased circulatory dehydroepiandrosterone and dehydroepiandrosterone-sulphate in first-episode schizophrenia: relationship to gender, aggression and symptomatology. Schizophrenia Research, 71(2–3), 427–434. https://doi.org/10.1016/j.schres.2004.03.005
Strous, R. D., Maayan, R., Lapidus, R., Stryjer, R., Lustig, M., Kotler, M., & Weizman, A. (2003). Dehydroepiandrosterone Augmentation in the Management of Negative, Depressive, and Anxiety Symptoms in Schizophrenia. Archives of General Psychiatry, 60(2), 133. https://doi.org/10.1001/archpsyc.60.2.133
Strous, R. D., Stryjer, R., Maayan, R., Gal, G., Viglin, D., Katz, E., Eisner, D., & Weizman, A. (2007). Analysis of clinical symptomatology, extrapyramidal symptoms and neurocognitive dysfunction following dehydroepiandrosterone (DHEA) administration in olanzapine treated schizophrenia patients: A randomized, double-blind placebo controlled trial. Psychoneuroendocrinology, 32(2), 96–105. https://doi.org/10.1016/j.psyneuen.2006.11.002
Tamagno, E., Guglielmotto, M., Bardini, P., Santoro, G., Davit, A., Di Simone, D., Danni, O., & Tabaton, M. (2003). Dehydroepiandrosterone reduces expression and activity of BACE in NT2 neurons exposed to oxidative stress. Neurobiology of Disease, 14(2), 291–301. https://doi.org/10.1016/S0969-9961(03)00131-1
van Waarde, A., Ramakrishnan, N. K., Rybczynska, A. A., Elsinga, P. H., Ishiwata, K., Nijholt, I. M., Luiten, P. G. M., & Dierckx, R. A. (2011). The cholinergic system, sigma-1 receptors and cognition. Behavioural Brain Research, 221(2), 543–554. https://doi.org/10.1016/j.bbr.2009.12.043
Wolkowitz, O. M., Kramer, J. H., Reus, V. I., Costa, M. M., Yaffe, K., Walton, P., Raskind, M., Peskind, E., Newhouse, P., Sack, D., De Souza, E., Sadowsky, C., & Roberts, E. (2003). DHEA treatment of Alzheimer’s disease: A randomized, double-blind, placebo-controlled study. Neurology, 60(7), 1071–1076. https://doi.org/10.1212/01.WNL.0000052994.54660.58
Yamada, S., Akishita, M., Fukai, S., Ogawa, S., Yamaguchi, K., Matsuyama, J., Kozaki, K., Toba, K., & Ouchi, Y. (2010). Effects of dehydroepiandrosterone supplementation on cognitive function and activities of daily living in older women with mild to moderate cognitive impairment. Geriatrics and Gerontology International, 10(4), 280–287. https://doi.org/10.1111/j.1447-0594.2010.00625.x