White Paper by Wei (Adelyn) Tsai
Reviewed by Greg Hollenbeck, Thia Hanania
Background
Pregnenolone (PREG) is a steroid precursor to many hormones in our body, including dehydroepiandrosterone (DHEA), progesterone, estrogen, testosterone, and cortisol (Akwa et al., 1991). It is synthesized from cholesterol and in the brain, it can be converted to pregnenolone sulfate (PREG-S), progesterone (PROG), and allopregnanolone (ALLO) (Fig.1) (Marx et al., 2011). PREG and PREG-S, however, decrease as ages, to as much as 60% by the age of 75 compared to the age of 35 years, and the hormones for which PREG is the precursor also diminish as people age (Roberts, 1995).
Fig. 1 Pregnenolone metabolism pathway. Pregnenolone is synthesized from cholesterol by the cytochrome P450 side-chain cleavage (P450scc). In the brain, pregnenolone can be converted into progesterone by 3b-hydroxysteroid dehydrogenase/isomerase (3β-HSD/isom), to pregnenolone sulfate by sulfotransferase, or to allopregnanolone by the serial actions of 3-b-HSD/isom, 5α -reductase, and 3α-Hydroxysteroid dehydrogenase (3α-HSD)(Marx et al., 2011)
Neurobiological functions
PREG is a neurosteroid with pleiotropic functions including the enhancement of learning and memory, neuritic outgrowth, and myelination, and antiapoptotic and anti-inflammatory effects (Marx et al., 2011). In addition, metabolites of PREG-S and ALLO are neurotransmitter receptor modulators. ALLO is an agonist of the γ-aminobutyric acid subtype A (GABAA) receptor (Wang et al., 2011), while PREG-S exhibits mixed GABA-agonistic/antagonistic features depending on the concentration (Majewska, 1992). PREG-S is also an agonist of the sigma-1 receptor and potentiates N-methyl-D-aspartate receptor (NMDA) receptors-mediated -mediated excitatory glutamatergic and noradrenergic neurotransmission (Noda, 2000). These functions underlie the potential therapeutic effects for many psychiatric and neurologic diseases.
Brain-health complaints
1) Schizophrenia and schizoaffective disorder
Aberrations in the hypothalamic–pituitary–adrenal (HPA) axis, neurotransmitter pathways, and neuroprotection underlie schizophrenia and schizoaffective disorder (SZ/SA)(Fig.2)(Cai et al., 2018). PREG has beneficial pleiotropic actions mentioned above that can improve schizophrenic symptoms. For example, through increasing downstream neurosteroids ALLO and PREG-S, which positively modulates GABAA receptor and NMDA receptors, respectively, PREG can ameliorate NMDA receptor hypofunction and GABA dysregulation in SZ/SA (Marx et al., 2011).
Fig. 2 Pregnenolone and its metabolites in schizophrenia (SZ). Disrupted neurosteroidgenesis may be caused by unknown SZ pathology, genetic liability, and/or environmental stress, and leads to abnormal levels of key neurosteroids, including pregnenolone (PREG), PREG sulfate, dehydroepiandrosterone (DHEA), DHEA sulfate, progesterone (PROG), and allopregnanolone (ALLO). Physiologically, abnormal levels of neurosteroids are associated with hypothalamic–pituitary–adrenal (HPA) axis dysregulation, increased anxiety and negative symptoms, and impaired cognition. ↑ indicates increased and ↓ indicates decreased. Modified from Cai et al., 2018.
Recent preclinical studies in a mouse model of schizophrenia (dopamine transporter knockout mouse) found that the administration of PREG and PREG-S could alleviate behavioral and cognitive deficits in these mice (Wong et al., 2015; Wong et al., 2012). Clinically, previous studies showed altered levels of PREG and its metabolites in SZ, but the directions of change are inconsistent (Cai et al., 2018; Marx et al., 2011). Clinical studies have shown evidence of PREG as a treatment for SZ/SA. In a pilot randomized, placebo-controlled, double-blind trial, patients who received adjunctive PREG for 8 weeks (fixed escalating doses to 500mg/day) had improved negative symptoms. After treatment, these patients had increased serum PREG and ALLO, which was significantly correlated with cognitive improvement. The PREG-treated group also had increased serum PREG-S (Marx et al., 2009). Subsequently, in another randomized controlled trial in 120 schizophrenic patients, PREG (maximum dose = 500mg qD for 4 weeks) was also given adjunctive to other antipsychotics. PREG, ALLO, and pregnanolone (a stereoisomer of ALLO with comparable GABAergic effects) serum levels increased after 8 weeks. Patients who received PREG had improved functional abilities and communication but not cognitive symptoms (Marx et al., 2014). Other studies using adjunctive PREG treatment in SZ/SA patients also showed improved negative symptoms, verbal and working memory, attention, extrapyramidal symptoms, reduced anxiety and executive function (Kardashev et al., 2018; Kashani et al., 2017; Kreinin et al., 2017; Ritsner et al., 2010, 2014; Savitz, 2010).
2) Stress-related disorder: Focus on anxiety and depression
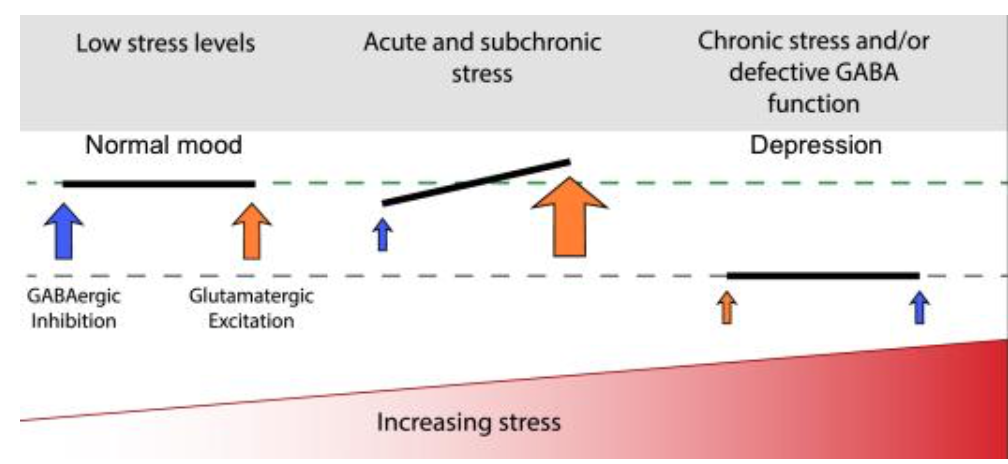
An imbalance of GABAergic and glutamatergic signaling induced by stress can result in anxiety disorder and depression (Meltzer-Brody & Kanes, 2020; Wieronska et al., 2011). Acute stress induces excessive glutamatergic transmission and impairs GABAergic transmission. As stress continues, both transmissions may be balanced but impaired, resulting in reduced neural connectivity (Fig.3). Importantly, neurosteroids level at GABAergic synapse alters in stressful conditions (Boero et al., 2020; Gunn et al., 2011), suggesting the importance of neurosteroids in stress-related illnesses. Indeed, in the serum, plasma, CSF, and brain patients with depression, a disease induced by chronic stress, the level and biosynthesis of ALLO, the metabolite of PREG, is reduced (Pinna, 2020).
Fig. 3 Impact of stress on the balance of GABAergic and glutamatergic transmission. Acute stress alters the balance between GABAergic and glutamatergic signaling, and further exposure to stress may result in a balanced but attenuated GABAergic and glutamatergic signaling, causing conditions such as depression (Meltzer-Brody & Kanes, 2020).
Much evidence so far has linked ALLO with treating anxiety and depression because of its observed reduction in patients and its biological actions. Decreased GABA levels and thus reduced GABAergic transmission underlie depression (Zorumski et al., 2013). As a GABAA receptor agonist, ALLO showed an antidepressant effect in mice forced swim test (Khisti et al., 2000). The ability of ALLO to influence the HPA axis also contributes to antidepressant effects. HPA axis activation and elevated vasopressin, corticotropin-releasing hormone (CRH), and adrenocorticotropic hormone (ACTH) are associated with depression (Holsboer, 2000). ALLO has a decremental effect on vasopressin, CRH, and ACTH level by potentiating the GABAergic inhibition on the HPA axis (Van Broekhoven & Verkes, 2003). Besides its antidepressant effect, ALLO is a potent anxiolytic agent by stimulating inhibitory GABAergic transmission (Reddy, 2010), which can balance the overstimulation of excitatory glutamatergic signaling in anxiety (Wieronska et al., 2011). By acting as a GABAA receptors agonist, ALLO and its precursor PROG, produced an anxiolytic effect in mice mirrored chamber test of anxiety (D.S. Reddy & Kulkarni, 1997). Therefore, PREG, through its metabolite ALLO, could be a potential antidepressant and anxiolytic.
In a study, healthy males were administered with 400mg PREG, which was administered to increased serum ALLO, and their brain activity in the amygdala and insula (emotion generation regions) and in dmPFC (emotion regulatory control region) were assessed while performing emotion appraisal task. Results showed that in response to emotional stimuli, the amygdala and insula activities were reduced and dmPFC activity and the connectivity between the amygdala and dmPFC enhanced. These results suggested increased serum ALLO was associated with reduced generation of negative emotion, increased emotional control, and reduced self-reported anxiety. Aberrant activities in these three regions are linked to anxiety pathology, so ALLO interventions, by acting on these regions, may be useful for treating anxiety disorder (Sripada et al., 2013). Interestingly, one randomized, double-blind, placebo-controlled trial showed PREG administration together with L-theanine, an amino acid found in green tea, and an analog to glutamine and glutamate, improved anxiety symptoms in schizophrenia and schizoaffective disorder patients (Kardashev et al., 2018).
A few clinical studies have shown PREG to be able to reduce depression. In a proof-of-concept trial done in 70 patients with bipolar disorder or recurrent major depressive disorder and history of substance abuse/dependence, it was found that PREG-treated group (titrated to 100mg/d, 8 weeks) had improved depressive symptoms (Osuji et al., 2010). In another study, Brown et al found improved depressive symptoms in bipolar disorder (BPD) patients after administration of PREG (titrated to 500mg/d) for 12 weeks. Interestingly the group also found a significant negative correlation between anxiety changes and ALLO level (Brown et al., 2014). Subsequently, the same group looked at the effect of PREG administration in microtubule-associated protein 2 (MAP2) levels in the blood. MAP2 stabilizes microtubules and promotes microtubule polymerization. MAP2 has dynamic functions in the brain, including regulating neuronal growth, differentiation, and plasticity (Johnson & Jope, 1992). Microtubules and MAPs are implicated in psychiatric disorders (Marchisella et al., 2016), and in humans, the MAP2 gene is associated with anhedonia (van Veen et al., 2012). PREG has been suggested to regulate microtubule and neuronal growth through MAP2 (Weng & Chung, 2016). In this study, it was found that, though not significant, MAP2 levels tended to be higher in BPD patients, and MAP2 changes (reduced over time, but not significantly different from placebo) correlated positively with changes in self-rated depressive symptom severity in the treatment but not in the placebo group. This suggested another possible mechanism of PREG to ameliorate depression via MAP2 (Daftary et al., 2017).
In conclusion, PREG is a neurosteroid with pleiotropic actions that enhance brain health. Its metabolites, PREG-S and ALLO, are neurotransmitter modulators important for the mechanisms of actions underlying the treatment effects of PREG. The present clinical studies show that 30-500mg/d of PREG is well tolerated and does not produce significant side effects. Importantly, based on currently available studies, PREG seems to be particularly useful as an adjunctive treatment for SZ/SA, alongside first- and second-generation antipsychotics, mood stabilizers, or L-theanine. Besides its effect on SZ/SA, PREG has also been suggested for treating anxiety disorder and depression, although more clinical studies will be needed to verify its efficacy.
References
Akwa, Y., Young, J., Kabbadj, K., Sancho, M. J., Zucman, D., Vourc’h, C., Jung-Testas, I., Hu, Z. Y., Le Goascogne, C., Jo, D. H., Corpéchot, C., Simon, P., Baulieu, E. E., & Robel, P. (1991). Neurosteroids: Biosynthesis, metabolism and function of pregnenolone and dehydroepiandrosterone in the brain. The Journal of Steroid Biochemistry and Molecular Biology, 40(1–3), 71–81. https://doi.org/10.1016/0960-0760(91)90169-6
Boero, G., Porcu, P., & Morrow, A. L. (2020). Pleiotropic actions of allopregnanolone underlie therapeutic benefits in stress-related disease. Neurobiology of Stress, 12(November 2019), 100203. https://doi.org/10.1016/j.ynstr.2019.100203
Brown, E. S., Park, J., Marx, C. E., Hynan, L. S., Gardner, C., Davila, D., Nakamura, A., Sunderajan, P., Lo, A., & Holmes, T. (2014). A randomized, double-blind, placebo-controlled trial of pregnenolone for bipolar depression. Neuropsychopharmacology, 39(12), 2867–2873. https://doi.org/10.1038/npp.2014.138
Cai, H., Cao, T., Zhou, X., & Yao, J. K. (2018). Neurosteroids in Schizophrenia: Pathogenic and Therapeutic Implications. Frontiers in Psychiatry, 9. https://doi.org/10.3389/fpsyt.2018.00073
Daftary, S., Yon, J.-M., Choi, E.-K., Kim, Y.-B., Bice, C., Kulikova, A., Park, J., & Sherwood Brown, E. (2017). Microtubule associated protein 2 in bipolar depression: Impact of pregnenolone. Journal of Affective Disorders, 218, 49–52. https://doi.org/10.1016/j.jad.2017.04.024
Gunn, B. G., Brown, A. G., Lambert, J. J., & Belelli, D. (2011). Neurosteroids and GABA A receptor interactions: A focus on stress. In Frontiers in Neuroscience (Vol. 5, Issue DEC). Frontiers Media SA. https://doi.org/10.3389/fnins.2011.00131
Holsboer, F. (2000). The Corticosteroid Receptor Hypothesis of Depression Clinical Evidence. Neuropsychopharmacology, 23(5), 477–501.
Johnson, G. V. W., & Jope, R. S. (1992). The role of microtubule-associated protein 2 (MAP-2) in neuronal growth, plasticity, and degeneration. Journal of Neuroscience Research, 33(4), 505–512. https://doi.org/10.1002/jnr.490330402
Kardashev, A., Ratner, Y., & Ritsner, M. S. (2018). Add-On Pregnenolone with L-Theanine to Antipsychotic Therapy Relieves Negative and Anxiety Symptoms of Schizophrenia: An 8-Week, Randomized, Double-Blind, Placebo-Controlled Trial. Clinical Schizophrenia & Related Psychoses, 12(1), 31–41. https://doi.org/10.3371/CSRP.KARA.070415
Kashani, L., Shams, N., Moazen-Zadeh, E., Karkhaneh-Yousefi, M.-A., Sadighi, G., Khodaie-Ardakani, M.-R., Rezaei, F., Rahiminejad, F., & Akhondzadeh, S. (2017). Pregnenolone as an adjunct to risperidone for treatment of women with schizophrenia: A randomized double-blind placebo-controlled clinical trial. Journal of Psychiatric Research, 94, 70–77. https://doi.org/10.1016/j.jpsychires.2017.06.011
Khisti, R. T., Chopde, C. T., & Jain, S. P. (2000). Antidepressant-like effect of the neurosteroid 3α-hydroxy-5α-pregnan-20-one in mice forced swim test. Pharmacology Biochemistry and Behavior, 67(1), 137–143. https://doi.org/10.1016/S0091-3057(00)00300-2
Kreinin, A., Bawakny, N., & Ritsner, M. S. (2017). Adjunctive Pregnenolone Ameliorates the Cognitive Deficits in Recent-Onset Schizophrenia: An 8-Week, Randomized, Double-Blind, Placebo-Controlled Trial. Clinical Schizophrenia & Related Psychoses, 10(4), 201–210. https://doi.org/10.3371/CSRP.KRBA.013114
Majewska, M. D. (1992). Neurosteroids: Endogenous bimodal modulators of the GABAA receptor mechanism of action and physiological significance. Progress in Neurobiology, 38(4), 379–394. https://doi.org/10.1016/0301-0082(92)90025-A
Marchisella, F., Coffey, E. T., & Hollos, P. (2016). Microtubule and microtubule associated protein anomalies in psychiatric disease. Cytoskeleton, 73(10), 596–611. https://doi.org/10.1002/cm.21300
Marx, C.E., Bradford, D. W., Hamer, R. M., Naylor, J. C., Allen, T. B., Lieberman, J. A., Strauss, J. L., & Kilts, J. D. (2011). Pregnenolone as a novel therapeutic candidate in schizophrenia: emerging preclinical and clinical evidence. Neuroscience, 191, 78–90. https://doi.org/10.1016/j.neuroscience.2011.06.076
Marx, Christine E., Lee, J., Subramaniam, M., Rapisarda, A., Bautista, D. C. T., Chan, E., Kilts, J. D., Buchanan, R. W., Wai, E. P., Verma, S., Sim, K., Hariram, J., Jacob, R., Keefe, R. S. E., & Chong, S. A. (2014). Proof-of-concept randomized controlled trial of pregnenolone in schizophrenia. Psychopharmacology, 231(17), 3647–3662. https://doi.org/10.1007/s00213-014-3673-4
Marx, Christine E, Keefe, R. S. E., Buchanan, R. W., Hamer, R. M., Kilts, J. D., Bradford, D. W., Strauss, J. L., Naylor, J. C., Payne, V. M., Lieberman, J. A., Savitz, A. J., Leimone, L. A., Dunn, L., Porcu, P., Morrow, A. L., & Shampine, L. J. (2009). Proof-of-Concept Trial with the Neurosteroid Pregnenolone Targeting Cognitive and Negative Symptoms in Schizophrenia. Neuropsychopharmacology, 34(8), 1885–1903. https://doi.org/10.1038/npp.2009.26
Meltzer-Brody, S., & Kanes, S. J. (2020). Allopregnanolone in postpartum depression: Role in pathophysiology and treatment. Neurobiology of Stress, 12(January), 100212. https://doi.org/10.1016/j.ynstr.2020.100212
Noda, Y. (2000). Neurosteroids Ameliorate Conditioned Fear Stress An Association with Sigma1 Receptors. Neuropsychopharmacology, 23(3), 276–284. https://doi.org/10.1016/S0893-133X(00)00103-2
Osuji, I. J., Vera-Bolaños, E., Carmody, T. J., & Brown, E. S. (2010). Pregnenolone for cognition and mood in dual diagnosis patients. Psychiatry Research, 178(2), 309–312. https://doi.org/10.1016/j.psychres.2009.09.006
Pinna, G. (2020). Allopregnanolone, the Neuromodulator Turned Therapeutic Agent: Thank You, Next? Frontiers in Endocrinology, 11(May), 1–6. https://doi.org/10.3389/fendo.2020.00236
Reddy, D.S., & Kulkarni, S. K. (1997). Differential anxiolytic effects of neurosteroids in the mirrored chamber behavior test in mice. Brain Research, 752(1–2), 61–71. https://doi.org/10.1016/S0006-8993(96)01447-3
Reddy, Doodipala Samba. (2010). Neurosteroids: endogenous role in the human brain and therapeutic potentials. Progress in Brain Research, 186, 113–137. https://doi.org/10.1016/B978-0-444-53630-3.00008-7
Reddy, Doodipala Samba, Kaur, G., & Kulkarni, S. K. (1998). Sigma (σ1) receptor mediated antidepressant-like effects of neurosteroids in the Porsolt forced swim test. NeuroReport, 9(13), 3069–3073. https://doi.org/10.1097/00001756-199809140-00028
Ritsner, M. S., Bawakny, H., & Kreinin, A. (2014). Pregnenolone treatment reduces severity of negative symptoms in recent-onset schizophrenia: An 8-week, double-blind, randomized add-on two-center trial. Psychiatry and Clinical Neurosciences, 68(6), 432–440. https://doi.org/10.1111/pcn.12150
Ritsner, M. S., Gibel, A., Shleifer, T., Boguslavsky, I., Zayed, A., Maayan, R., Weizman, A., & Lerner, V. (2010). Pregnenolone and dehydroepiandrosterone as an adjunctive treatment in schizophrenia and schizoaffective disorder: an 8-week, double-blind, randomized, controlled, 2-center, parallel-group trial. The Journal of Clinical Psychiatry, 71(10), 1351–1362. https://doi.org/10.4088/JCP.09m05031yel
Roberts, E. (1995). Pregnenolone—from selye to Alzheimer and a model of the pregnenolone sulfate binding site on the GABAA receptor. Biochemical Pharmacology, 49(1), 1–16. https://doi.org/10.1016/0006-2952(94)00258-N
Savitz, A. J. (2010). Multi-year continuation study of pregnenolone in patients with schizophrenia. Biol Psychiatry, 67(9S), 225.
Schüle, C., Eser, D., Baghai, T. C., Nothdurfter, C., Kessler, J. S., & Rupprecht, R. (2011). Neuroactive steroids in affective disorders: target for novel antidepressant or anxiolytic drugs? Neuroscience, 191, 55–77. https://doi.org/10.1016/j.neuroscience.2011.03.025
Sripada, R. K., Marx, C. E., King, A. P., Rampton, J. C., Ho, S. S., & Liberzon, I. (2013). Allopregnanolone Elevations Following Pregnenolone Administration Are Associated with Enhanced Activation of Emotion Regulation Neurocircuits. Biological Psychiatry, 73(11), 1045–1053. https://doi.org/10.1016/j.biopsych.2012.12.008
Urani, A., Roman, F. J., Phan, V.-L., Su, T.-P., & Maurice, T. (2001). The Antidepressant-Like Effect Induced by σ1-Receptor Agonists and Neuroactive Steroids in Mice Submitted to the Forced Swimming Test. JJ Pharmacol Exp Ther, 298((3)), 1269–1279.
Van Broekhoven, F., & Verkes, R. J. (2003). Neurosteroids in depression: A review. Psychopharmacology, 165(2), 97–110. https://doi.org/10.1007/s00213-002-1257-1
van Veen, T., Goeman, J. J., Monajemi, R., Wardenaar, K. J., Hartman, C. A., Snieder, H., Nolte, I. M., Penninx, B. W. J. H., & Zitman, F. G. (2012). Different gene sets contribute to different symptom dimensions of depression and anxiety. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 159B(5), 519–528. https://doi.org/10.1002/ajmg.b.32058
Wang, M., Rahman, M., Strömberg, J., Lundgren, P., Haage, D., Johansson, I.-M., & Bäckström, T. (2011). Neurosteroids and GABA-A Receptor Function. Frontiers in Endocrinology, 2, 44. https://doi.org/10.3389/fendo.2011.00044
Weng, J.-H., & Chung, B. (2016). Nongenomic actions of neurosteroid pregnenolone and its metabolites. Steroids, 111, 54–59. https://doi.org/10.1016/j.steroids.2016.01.017
Wieronska, J. M., Stachowicz, K., Nowak, G., & Pilc, A. (2011). The Loss of Glutamate-GABA Harmony in Anxiety Disorders. In Anxiety Disorders. InTech. https://doi.org/10.5772/19919
Wong, P, Sze, Y., Chang, C. C. R., Lee, J., & Zhang, X. (2015). Pregnenolone sulfate normalizes schizophrenia-like behaviors in dopamine transporter knockout mice through the AKT/GSK3β pathway. Translational Psychiatry, 5(3), e528–e528. https://doi.org/10.1038/tp.2015.21
Wong, Peiyan, Chang, C. C. R., Marx, C. E., Caron, M. G., Wetsel, W. C., & Zhang, X. (2012). Pregnenolone Rescues Schizophrenia-Like Behavior in Dopamine Transporter Knockout Mice. PLoS ONE, 7(12), e51455. https://doi.org/10.1371/journal.pone.0051455
Zorumski, C. F., Paul, S. M., Izumi, Y., Covey, D. F., & Mennerick, S. (2013). Neurosteroids, stress and depression: Potential therapeutic opportunities. Neuroscience & Biobehavioral Reviews, 37(1), 109–122. https://doi.org/10.1016/j.neubiorev.2012.10.005