White Paper by Wei (Adelyn) Tsai
Reviewed byGreg Hollenbeck, Thia Hanania
ARA290 is an 11 amino acid peptide derived from the helix B of erythropoietin (EPO), which is a cytokine that is synthesized to trigger anti-inflammatory and antiapoptotic processes in response to tissue injury, hypoxia, or metabolic stress (Michael Brines & Cerami, 2012; Dahan et al., 2016). Although exogenous EPO had shown to be effective for experimental models of brain injuries, in humans, high concentrations of EPO are needed for tissue protection, which may cause constant activation of its hematopoietic and vascular activities, including strong pro‐coagulant and hemodynamic effects (Fig.1)(Brines & Cerami, 2008). These effects produced serious thrombotic complications in clinical trials (Corwin et al., 2007).
Fig.1 Pharmacokinetic analyses of bolus intravenous doses of rhEPO administered to normal volunteers. EPO has a higher affinity to receptors involved in the hematopoiesis, so less dosage (~100IU kg-1) is required. In contrast, the affinity of EPO to tissue-protective receptors is much lower. Therefore, a much higher dosage (~500IU kg-1) is necessary for tissue protection. In humans, this high dosage, which is way above the minimum dosage for hematopoiesis, could cause hematopoietic and thrombotic side effects. Moreover, EPO has a long plasma-half life so it can cause continuous activation of hematopoiesis processes. The black inverted triangle indicates the estimated peak rhEPO concentration achieved in clinical studies in stroke and cognition (Brines & Cerami, 2008).
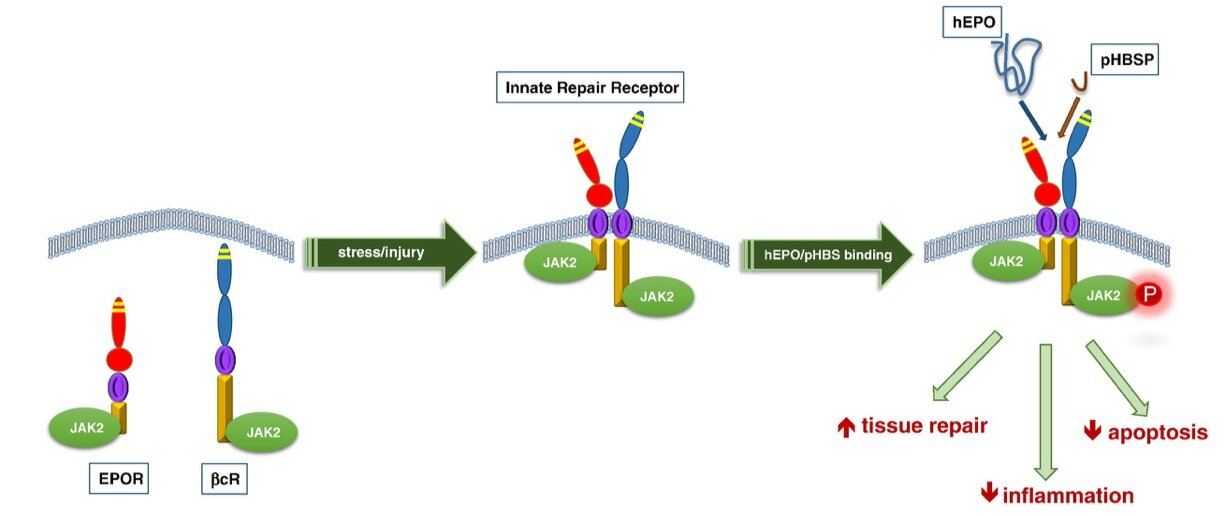
Importantly, ARA290 is neuroprotective and tissue-protective without stimulating erythropoiesis and pro-coagulation, therefore eliminating the potential of causing hematopoietic and thrombotic side effects (Michael Brines et al., 2008). It acts on the innate repair receptor (IRR), leading to the initiation of different signaling cascades producing protective and reparative effects (Collino et al., 2015) (Fig.2&3). ARA290 could also inhibit a nociceptor, TRPV1 channel, and relieve the capsaicin-induced mechanical pain (Zhang et al., 2016). Intriguingly, although ARA290 has a very short half-life of ~2mins, it produced persistent biological effects over hours to days. Therefore, pharmacokinetics does not play a main role in the efficacy of ARA290. The most likely explanation is that its binding to the IRR triggers sustained cascades leading to prolonged biological effects (Niesters et al., 2013).
Fig.2 The innate repair receptor (IRR) system. IRR is a member of the type I cytokine receptor family and is composed of β common receptor (βcR) and EPO receptor subunits. Upon cellular stress or tissue injuries, EPOR and βcR subunits are translocated to the membrane, forming the IRR. The endogenous ligand, hyposialated EPO, and exogenous ligand, ARA290 (pyroglutamate helix B surface peptide [pHBSP]) can bind to IRR, causing the phosphorylation of Janus kinase 2 (JAK2), which subsequently initiates multiple signaling pathways that improve tissue repair, reduce inflammation and apoptosis (Collino et al., 2015).
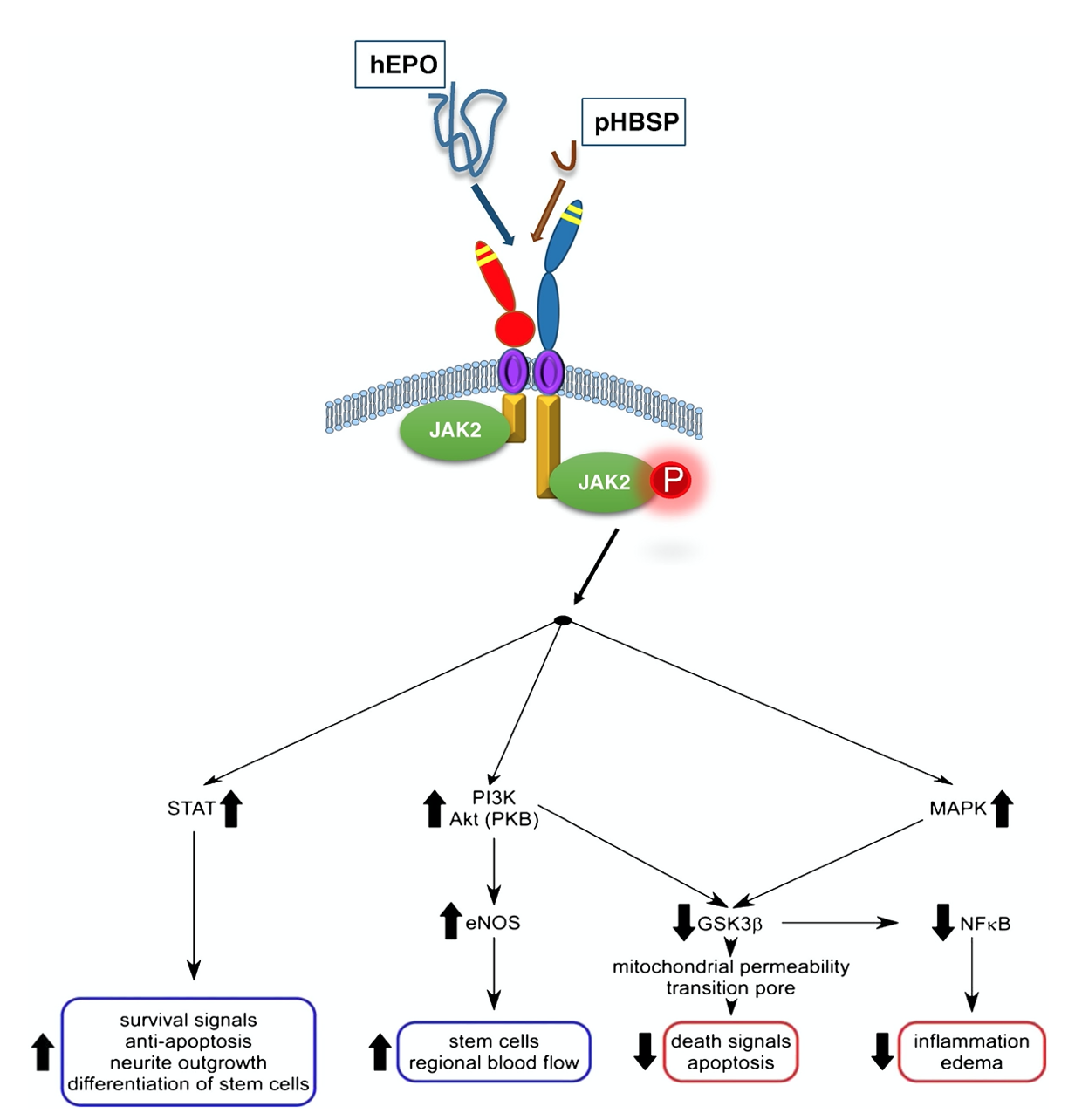
Fig.3 Multiple intracellular signaling pathways are implicated in the biological activity of ARA290.
ARA290 can cross the blood-brain barrier rapidly (Niesters et al., 2013). Most studies focus on the effects of ARA290 on pain. For example, in rats and mice models of spared nerve injury, ARA290 produced relief of allodynia by acting on βcR (Swartjes et al., 2011) and suppressing spinal microglial activation. These suggested that ARA290 induced relief of neuropathy via immune suppression (Swartjes et al., 2014).
Similarly, in humans, several studies reported the beneficial effects of ARA290 for treating neuropathic pain, along with other symptoms. Several studies investigated whether ARA290 could alleviate symptoms in sarcoidosis, an inflammatory organ disease characterized by small nerve fibers (SNF) dysfunction. The patients often have pain characterized by dysesthesia and allodynia, and loss of cutaneous sensation and autonomic functions. Heij et al used 2mg of ARA290 and administered intravenously every other day for 4 weeks. They found reduced severity of symptoms, particularly neuropathic symptoms and autonomic dysfunction, and improved quality of life in the pain and physical functioning dimensions (Heij et al., 2012). Two other studies (Culver et al., 2017; Dahan et al., 2013) used subcutaneous administration of 4mg/d for 28 days. Improvement in pain and corneal small nerve fiber density, which quantifies SNF, was reported. Dahan et al also found improved exercise capacity, which is also often impaired in sarcoidosis patients, and increased threshold of temperature sensitivity. Since thermal sensory function is related to small fiber, a greater threshold indicated better restoration of the SNF sensory functions. In line with these findings, Culver et al also found increased skin nerve fiber. Therefore, ARA290 is therapeutic for sarcoidosis.
ARA290 may also be therapeutic for neuropathic pain associated with type 2 diabetes. In a study using subcutaneous administration of 4mg/d for 28 days in type 2 diabetic patients, it was found that patients had improved neuropathic pain and increased corneal small nerve fiber density in patients having abnormally reduced fiber at baseline. These two outcomes were correlated, suggesting the relationship between SNF density and neuropathic symptoms. Lastly, patients also had improved quality of life primarily in vitality (energy/fatigue) dimensions (Brines et al., 2014).
Besides treating neuropathic pain, other possible neurological therapeutic effects of ARA290 have been explored. ARA290 suppresses inflammation, together with other effects, that contribute to the improvement of symptomologies in several disease models. In autoimmune encephalomyelitis rats, which model multiple sclerosis, ARA290 was able to downregulate proinflammatory cytokines expression in the spinal cord, inhibit lymphocyte proliferation and alter proliferation of T cells. All these changes produced anti-inflammatory effects that reduced symptom severity and duration in these rats (Chen et al., 2014). Similarly, in a rat model of autoimmune neuritis, which mimics the Guillain–Barré Syndrome, ARA290 inhibited lymphocyte proliferation and inflammatory macrophage activation and triggered T cells proliferation in the direction favoring anti-inflammation. Notably, ARA290 also exerted anti-inflammatory effects and enhanced remyelination on Schwann cells, which are often demyelinated in autoimmune neuritis due to inflammation (Liu et al., 2014). In mild traumatic brain injury rat model, ARA290 reduced activated inflammatory cells as well as improved cognitive functions (Robertson et al., 2013). Administration of ARA290 in epileptic rats increased hippocampal cell proliferation and neurogenesis, thereby improving cognitive deficits associated with epilepsy (Seeger et al., 2011). Interestingly, EPO has been shown to be effective for treating cognitive deficits associated with schizophrenia, bipolar disorder, and major depression, suggesting the therapeutic potential of ARA290 for neuropsychiatric disorders (Li et al., 2018). Cerit et al tested if ARA290 had antidepressant properties in a group of healthy participants. Although the study did not find evidence to support that ARA290 had antidepressant properties, it did find that ARA290 could modulate general emotional processing, therefore encouraging further study using higher doses and longer and repetitive treatment (Cerit et al., 2015). Altogether, these studies suggested that ARA290 has strong anti-inflammatory effects that can be extended to treat other neurologic diseases. It is also cytoprotective on different central and peripheral neurons. Ultimately, the biological changes exerted by ARA290 may affect disease symptoms as well as subjects’ cognition.
ARA290 is manufactured by Araim Pharmaceuticals Inc. and is not yet available over the counter. It has so far only been proven to be effective for neuropathic pain in humans and has received US Orphan Drug and Fast Track designations for the treatment of neuropathic pain in patients with sarcoidosis (Araim Pharmaceuticals’ Cibinetide (ARA 290) Regenerates Small Nerve Fibers and Improves Neuropathic Clinical Symptoms in the Orphan Disease of Sarcoidosis – Araim Pharmaceuticals, 2017). Still, its effects on other diseases are waited to be tested and therefore should be used with caution for treatments other than sarcoidosis. Previous studies in sarcoidosis and type 2 diabetic patients demonstrated that subcutaneous 4mg/d was effective and produced no major side effects. In conclusion, ARA290 is a useful peptide acting on IRR and generating protective and reparative effects. It seems to be particularly useful for inflammation-associated neurologic diseases, while its effects on cognitive functions still need further tests and elucidation.
References
Araim Pharmaceuticals’ Cibinetide (ARA 290) Regenerates Small Nerve Fibers and Improves Neuropathic Clinical Symptoms in the Orphan Disease of Sarcoidosis – Araim Pharmaceuticals. (2017). http://www.araimpharma.com/cibinetide-ara-290-regenerates-small-nerve-fibers-neuropathic-orphan-disease-sarcoidosis/
Brines, M., & Cerami, A. (2008). Erythropoietin-mediated tissue protection: Reducing collateral damage from the primary injury response. In Journal of Internal Medicine (Vol. 264, Issue 5, pp. 405–432). John Wiley & Sons, Ltd. https://doi.org/10.1111/j.1365-2796.2008.02024.x
Brines, Michael, & Cerami, A. (2012). The receptor that tames the innate immune response. In Molecular medicine (Cambridge, Mass.) (Vol. 18, Issue 1, pp. 486–496). The Feinstein Institute for Medical Research. https://doi.org/10.2119/molmed.2011.00414
Brines, Michael, Dunne, A. N., van Velzen, M., Proto, P. L., Ostenson, C. G., Kirk, R. I., Petropoulos, I. N., Javed, S., Malik, R. A., Cerami, A., & Dahan, A. (2014). ARA 290, a nonerythropoietic peptide engineered from erythropoietin, improves metabolic control and neuropathic symptoms in patients with type 2 diabetes. Molecular Medicine (Cambridge, Mass.), 20, 658–666. https://doi.org/10.2119/molmed.2014.00215
Brines, Michael, Patel, N. S. A., Villa, P., Brines, C., Mennini, T., De Paola, M., Erbayraktar, Z., Erbayraktar, S., Sepodes, B., Thiemermann, C., Ghezzi, P., Yamin, M., Hand, C. C., Xie, Q. W., Coleman, T., & Cerami, A. (2008). Nonerythropoietic, tissue-protective peptides derived from the tertiary structure of erythropoietin. Proceedings of the National Academy of Sciences of the United States of America, 105(31), 10925–10930. https://doi.org/10.1073/pnas.0805594105
Cerit, H., Veer, I. M., Dahan, A., Niesters, M., Harmer, C. J., Miskowiak, K. W., Rombouts, S. A. R. B., & Van der Does, W. (2015). Testing the antidepressant properties of the peptide ARA290 in a human neuropsychological model of drug action. European Neuropsychopharmacology, 25(12), 2289–2299. https://doi.org/10.1016/j.euroneuro.2015.09.005
Chen, H., Luo, B., Yang, X., Xiong, J., Liu, Z., Jiang, M., Shi, R., Yan, C., Wu, Y., & Zhang, Z. (2014). Therapeutic effects of nonerythropoietic erythropoietin analog ARA290 in experimental autoimmune encephalomyelitis rat. Journal of Neuroimmunology, 268(1–2), 64–70. https://doi.org/10.1016/j.jneuroim.2014.01.006
Collino, M., Thiemermann, C., Cerami, A., & Brines, M. (2015). Flipping the molecular switch for innate protection and repair of tissues: Long-lasting effects of a non-erythropoietic small peptide engineered from erythropoietin. Pharmacology and Therapeutics, 151, 32–40. https://doi.org/10.1016/j.pharmthera.2015.02.005
Corwin, H. L., Gettinger, A., Fabian, T. C., May, A., Pearl, R. G., Heard, S., An, R., Bowers, P. J., Burton, P., Klausner, M. A., & Corwin, M. J. (2007). Efficacy and safety of epoetin alfa in critically ill patients. New England Journal of Medicine, 357(10), 965–976. https://doi.org/10.1056/NEJMoa071533
Culver, D. A., Dahan, A., Bajorunas, D., Jeziorska, M., van Velzen, M., Aarts, L. P. H. J., Tavee, J., Tannemaat, M. R., Dunne, A. N., Kirk, R. I., Petropoulos, I. N., Cerami, A., Malik, R. A., & Brines, M. (2017). Cibinetide improves corneal nerve fiber abundance in patients with sarcoidosis-associated small nerve fiber loss and neuropathic pain. Investigative Ophthalmology and Visual Science, 58(6), BIO52–BIO60. https://doi.org/10.1167/iovs.16-21291
Dahan, A., Brines, M., Niesters, M., Cerami, A., & van Velzen, M. (2016). Targeting the innate repair receptor to treat neuropathy. PAIN Reports, 1(1), e566. https://doi.org/10.1097/pr9.0000000000000566
Dahan, A., Dunne, A., Swartjes, M., Proto, P. L., Heij, L., Vogels, O., van Velzen, M., Sarton, E., Niesters, M., Tannemaat, M. R., Cerami, A., & Brines, M. (2013). ARA 290 improves symptoms in patients with sarcoidosis-associated small nerve fiber loss and increases corneal nerve fiber density. Molecular Medicine, 19(1), 334–345. https://doi.org/10.2119/molmed.2013.00122
Heij, L., Niesters, M., Swartjes, M., Hoitsma, E., Drent, M., Dunne, A., Grutters, J. C., Vogels, O., Brines, M., Cerami, A., & Dahan, A. (2012). Safety and Efficacy of ARA 290 in Sarcoidosis Patients with Symptoms of Small Fiber Neuropathy: A Randomized, Double-Blind Pilot Study. Molecular Medicine, 18(11), 1430–1436. https://doi.org/10.2119/molmed.2012.00332
Li, X. Bin, Zheng, W., Ning, Y. P., Cai, D. Bin, Yang, X. H., Ungvari, G. S., Ng, C. H., Wang, C. Y., & Xiang, Y. T. (2018). Erythropoietin for Cognitive Deficits Associated with Schizophrenia, Bipolar Disorder, and Major Depression: A Systematic Review. In Pharmacopsychiatry (Vol. 51, Issue 3, pp. 100–104). Georg Thieme Verlag. https://doi.org/10.1055/s-0043-114670
Liu, Y., Luo, B., Han, F., Li, X., Xiong, J., Jiang, M., Yang, X., Wu, Y., & Zhang, Z. (2014). Erythropoietin-Derived Nonerythropoietic Peptide Ameliorates Experimental Autoimmune Neuritis by Inflammation Suppression and Tissue Protection. PLoS ONE, 9(3), e90942. https://doi.org/10.1371/journal.pone.0090942
Niesters, M., Swartjes, M., Heij, L., Brines, M., Cerami, A., Dunne, A., HoitsM.A., E., & Dahan, A. (2013). The erythropoietin analog ARA 290 for treatment of sarcoidosis-induced chronic neuropathic pain. Expert Opinion on Orphan Drugs, 1(1), 77–87. https://doi.org/10.1517/21678707.2013.719289
Robertson, C. S., Garcia, R., Gaddam, S. S. K., Grill, R. J., Cerami Hand, C., Tian, T. S., & Hannay, H. J. (2013). Treatment of Mild Traumatic Brain Injury with an Erythropoietin-Mimetic Peptide. Journal of Neurotrauma, 30(9), 765–774. https://doi.org/10.1089/neu.2012.2431
Seeger, N., Zellinger, C., Rode, A., Roloff, F., Bicker, G., Russmann, V., Fischborn, S., Wendt, H., & Potschka, H. (2011). The erythropoietin-derived peptide mimetic pHBSP affects cellular and cognitive consequences in a rat post-status epilepticus model. Epilepsia, 52(12), 2333–2343. https://doi.org/10.1111/j.1528-1167.2011.03302.x
Swartjes, M., Morariu, A., Niesters, M., Brines, M., Cerami, A., Aarts, L., & Dahan, A. (2011). ARA290, a Peptide Derived from the Tertiary Structure of Erythropoietin, Produces Long-term Relief of Neuropathic Pain. Anesthesiology, 115(5), 1084–1092. https://doi.org/10.1097/ALN.0b013e31822fcefd
Swartjes, M., van Velzen, M., Niesters, M., Aarts, L., Brines, M., Dunne, A., Cerami, A., & Dahan, A. (2014). ARA 290, a Peptide Derived from the Tertiary Structure of Erythropoietin, Produces Long-Term Relief of Neuropathic Pain Coupled with Suppression of the Spinal Microglia Response. Molecular Pain, 10, 1744-8069-10–13. https://doi.org/10.1186/1744-8069-10-13
Zhang, W., Yu, G., & Zhang, M. (2016). ARA 290 relieves pathophysiological pain by targeting TRPV1 channel: Integration between immune system and nociception. Peptides, 76, 73–79. https://doi.org/10.1016/j.peptides.2016.01.003