White Paper by Ben Kronz
Peer-reviewed by Kritika Phatak
Body protein compound 157 (BPC-157) has shown to have a wide range of effects on the body, this is due to its therapeutic effects on the stomach and intestinal tract. [26,27,30] It has been shown to be an anti-ulcer, alleviates inflammatory bowel syndrome, stimulates tendon and tissue healing, promotes angiogenesis, and even has an effect on brain health. [1,14,15,9,28, 29] BPC-157 could potentially be an effective therapy for aging populations as the mucous membrane loses integrity with increased age. [16,31,32]
BPC-157 and Angiogenesis
Angiogenesis plays a large role in the aging process and if reversed could have a significant effect on longevity and overall health. Researchers have found that BPC-157 is a powerful tool for healthy aging by the upregulation of angiogenesis.[33,34] The process of Angiogenesis is the growth of new capillaries in an organism. [1] As we age the risk of having an ischemia doubles every 10 years after turning 55. [2,35,36] This is important because the angiogenesis of blood capillaries is important for the recovery from ischemia. Also, the downregulation of angiogenesis has shown to cause heart problems, if there is an imbalance between cardiovascular tissue and blood vessels, this has shown to increase the chances of heart failure. [6, 37,38,39] If Angiogenesis can be upregulated then the aging health and life expectancy could potentially be extended.
Mechanisms of Angiogenesis
As people age, there is a loss of function in many parts of the angiogenic pathway that are also important in the aging process. [40,41] Researchers have found that as we age there is a significant drop off in the Hypoxia-inducible factor - 1ɑ (HIF-1ɑ), PCG-1ɑ, and endothelial nitric oxide synthase (eNOS)[3,42,43,44]. These are all very important in the angiogenic pathway (figure 1), HIF-1ɑ and PCG-1ɑ promote Vascular endothelial growth factors (VEGF) which is one of the crucial growth factors that regulate capillary growth.[45,46] As we age the levels of HIF-1ɑ and PCG-1ɑ decrease.[4,47] eNOS is also important for this process as it produces nitric oxide which directly activates the angiogenic process, and as we age the eNOS can become uncoupled from its cofactor tetrahydrobiopterin, this results in the loss of NO in the endothelial cells of the capillaries. [5,48,49] The process of angiogenesis is clear and can be reversed by peptides such as BPC-157. [50]
Figure 1: The Angiogenic Pathway [3]
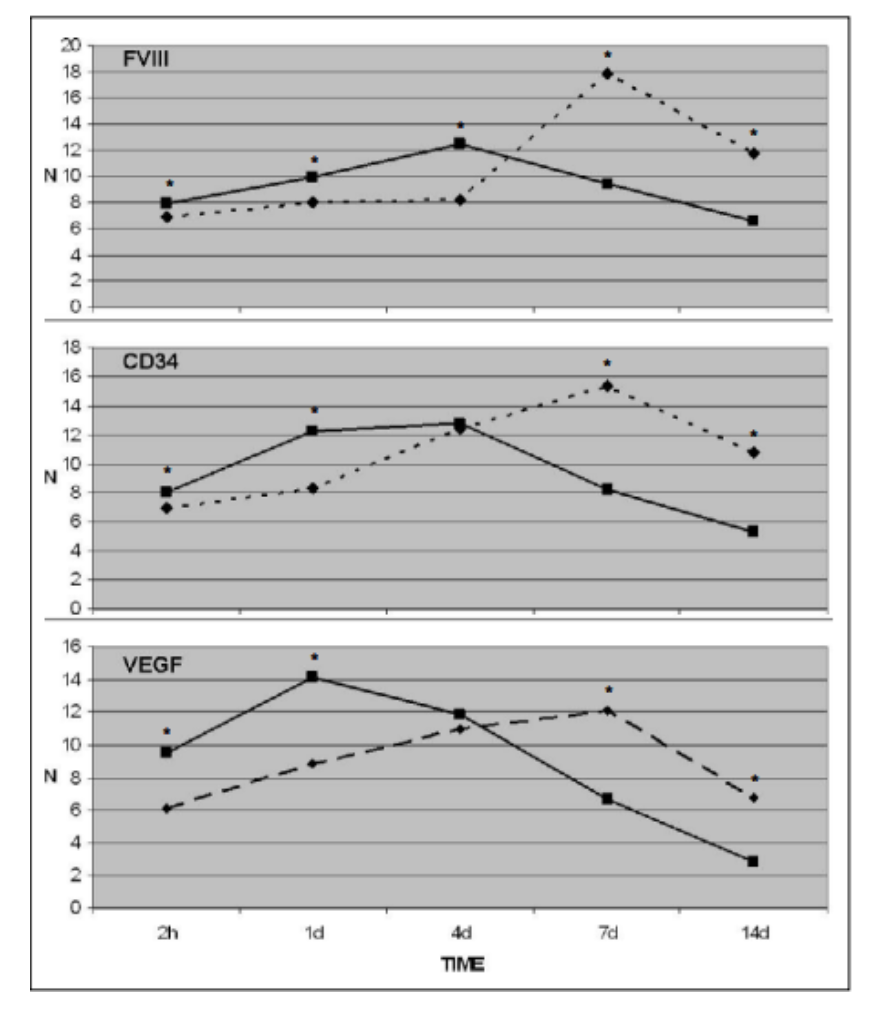
BPC-157 has shown to have a wide range of effects on the body, one of the most important for longevity is its role in angiogenesis. L, Bric et al looked into the effects of angiogenesis on muscles and tendons on mice that had an induced injury. He found that there was a significant increase in the angiogenic response in mice with BPC-157.[51] Because of this process, the mice had a faster muscle and tendon repair compared to the control. [4] This happens because the BPC-157 upregulates some of the essential parts of the Angiogenic pathway that we have previously discussed. It has shown to upregulate VEGF, CD34, and FVIII in the rats that were given BPC-157. [4,52,] We have looked into the effects of VEGF in the role of angiogenesis as it upregulates the eNOS enzyme and, therefore, the amount of NO in the organism for capillary regeneration. [4,52] BPC-157 has also shown to have a direct effect on the levels of NO in the organism as well; it has proved to deactivate the effects of the NOS-inhibitors and the NO-precursor. [7,54] These have direct effects on angiogenesis.[55,56] CD34 and FVIII show to be expressed in the formation of endothelial cells [8,53,57] A higher expression of CD34 and FVIII indicate that the process of angiogenesis is activated. [58]
Figure 2: The levels of indicators and promoters of angiogenesis over time in muscle tissue. Legend: N- number of positive elements, full line- BPC 157 treated animals; broken line- controls; time- time after transection; *- statistically significant difference (p<0.05). [4]
BPC 157 and Healing Tissues
The upregulation of Angiogenesis can have significant effects on the healing process of crushed muscle or tendons. [59,60] Researchers found that there is a significant difference in the rate at which muscles and tendons can repair themselves when on BPC-157. [4,61] The muscles were able to get back to normal faster both macroscopically (no post-injury leg contracture) and microscopically as enzyme activity in the muscle cells were able to get back to normal levels more quickly. [4] This could be very effective in helping the aging population bounce back from muscle and tendon damage as well as recovery of stroke as the peptide upregulates angiogenesis. [62,63]
BPC-157 and the Brain
The role of BPC-157 on the brain is quite intriguing; we have noted that BPC-157 has a wide range of effects on the body. [26,64] We will focus on its role in the GI tract and how gut health affects the brain. The Gut-Brain Axis (GBA) is how BPC-157 has a therapeutic effect on the brain through the gut. It is a two-way communication center between the central and enteric nervous systems. [9,65] This links the emotional and neurological regions of the brain to the gut and its health. [66] This functions as a way to keep the gastric juices in the stomach balanced and helps maintain the overall homeostasis of the GI tract. [67] This has also shown to link to higher cognitive functions like emotion and motivation. [9,65,66] This is all mediated by the vagus nerve connecting the enteric nervous system and the central nervous system. [68] This crosstalk between the brain and the gut is the mechanism in which BPC-157 specifically targets the gut which therefore affects the brain. [10] BPC-157 is a potent anti-ulcer agent and cytoprotective agent for the gut. [69] The effects on the brain are accomplished by maintaining GI mucous integrity. [11,70]
Figure 1: The Brain-Gut Axis/ How the CNS and the GI communicate.[22]
Studies show that BPC-157 regulates the serotonergic and dopaminergic systems by helping the GI tract return to homeostasis. [71] This is accomplished by stimulating nerve regeneration of damaged neurons from the overstimulation of neurotransmitters. [11] There are multiple ways in which BPC-157 stimulates nerve health and nerve regeneration. BPC-157 stimulates the Egr-1 gene, NAB2, and JAK-2 in the brain. [9,72,73,74] The Erg-1 gene product produces a zinc finger-type enzyme and has been identified in nerve growth factor-induced PC12 cells in rat fibroblasts. [12] This PC12 cell line is a model for cell differentiation in Rat fibroblasts and showed to have an upregulation of Erg-1 in the cell nucleus which shows its probable link to nerve regeneration. [12.75,76] NAB2 has also proved to be important for the brain in both the myelination of neurons and gene transcription. It is essential in transcription because it controls the length of the poly-A tail of the 3’ end of mRNA; this is important for the preservation of mRNA and the production of proteins in the brain. [13,77,78] This pathway is essential as it prevents demyelination, the result of diseases like Multiple sclerosis (MS). [17] The JAK-2 pathways are involved in cytokine regulation and immune function, the mechanism is not yet clear. Still, it has shown to be important as mouse models with the JAK-2 gene knocked out the mouse died around the 8th week of its life, compared to the average lifespan of a mouse is two years. [18]
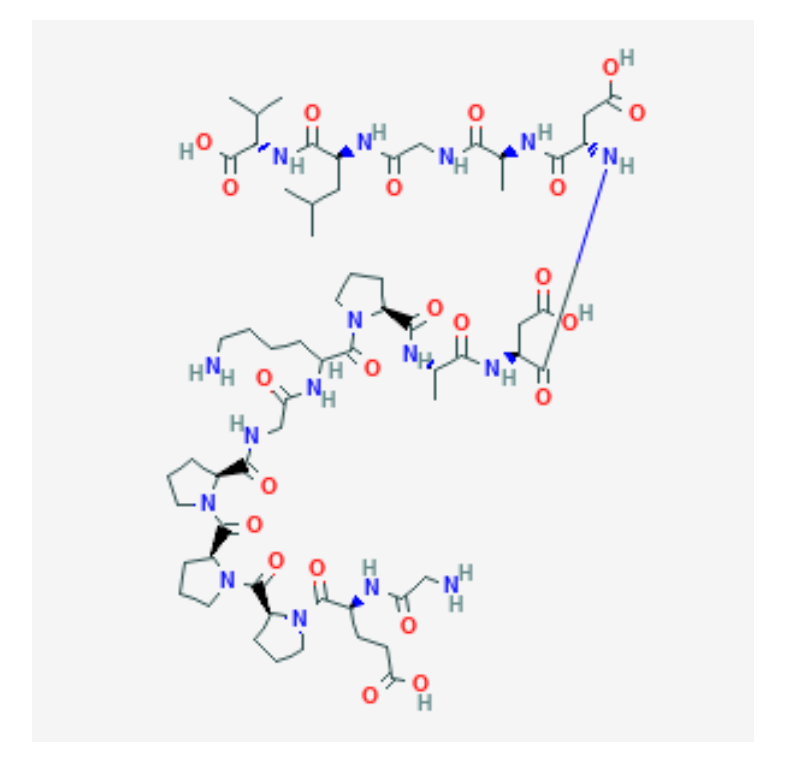
Figure 4: The molecular structure of peptide BPC-157. [23]
These pathways are essential for the brain and its higher level of processing, as they have shown to balance the central/neural dopaminergic and serotonergic systems. [19,20,79] It has been shown to specifically induce the release of serotonin in specific regions of the brain. [80] This can help repair these systems as aging populations have damaged dopaminergic and serotonergic systems. Dopamine levels drop off by 10% per decade since young adulthood. [21,81,82] This is associated with a decrease in cognitive and motor function. [83,84] The receptors for dopamine decrease as the levels of dopamine decrease. [85] This is significant as BPC-157 has shown to rebalance the receptor level of dopamine in the brain. [9] Serotonin levels also drop off as we age, [86] resulting in a decrease in synaptic plasticity and neurogenesis. [87] This is important for the efficiency of the brain as we grow older as the loss of synaptic plasticity inhibits synaptic pruning. [21,88] The therapeutic effects reach past aging and dopaminergic and serotonergic homeostasis. Research has shown it to be useful for repairing lesions in the stomach caused by alcohol, insulin, or NSAIDs. [9] It has also proved to be beneficial for Parkinson’s patients as it interacts with the MPTP, which causes permanent damage to dopaminergic regions in the basal ganglia. [24] It has also been shown that BPC-157 reverses damage caused by amphetamines in the central dopaminergic system, such as neuronal damage, reduced dopaminergic activity, and dopaminergic vesicle depletion. [9,25] Another application of BPC-157 is in aging populations with MS. The NAB2 pathway seems to interact with the neurons to reduce demyelination in the brain, which explains the reduction in the symptoms of rats who had induced MS by cuprizone. [9] BPC-157 has a wide range of effects on the body and can be very useful in a clinical setting for those with the brain, GI, or blood vessel problems that could benefit from this therapy.
References
[1] Folkman, J. (1984). Angiogenesis. Developments in Cardiovascular Medicine Biology of Endothelial Cells, 412-428. doi:10.1007/978-1-4613-2825-4_42
[2] Yousufuddin, M., & Young, N. (2019). Aging and ischemic stroke. Aging, 11(9), 2542–2544. https://doi.org/10.18632/aging.101931
[3] Lähteenvuo, J., & Rosenzweig, A. (2012). Effects of Aging on Angiogenesis. Circulation Research, 110(9), 1252-1264. doi:10.1161/circresaha.111.246116
[4]Novinscak T, Brcic L, Staresinic M, et al. Gastric pentadecapeptide BPC 157 as an effective therapy for muscle crush injury in the rat. Surg Today. 2008;38(8):716-725. doi:10.1007/s00595-007-3706-2
[5] Vásquez-Vivar, J., Kalyanaraman, B., Martásek, P., Hogg, N., Masters, B. S., Karoui, H., . . . Pritchard, K. A. (1998). Superoxide generation by endothelial nitric oxide synthase: The influence of cofactors. Proceedings of the National Academy of Sciences, 95(16), 9220-9225. doi:10.1073/pnas.95.16.9220
[6] Isner, J. M., & Losordo, D. W. (1999). Therapeutic angiogenesis for heart failure. Nature Medicine, 5(5), 491-492. doi:10.1038/8374
[7] Grabarevic, Z., Tisljar, M., Artukovic, B., Bratulic, M., Dzaja, P., Seiwerth, S., . . . Kos, J. (1997). The influence of BPC 157 on nitric oxide agonist and antagonist induced lesions in broiler chicks. Journal of Physiology-Paris, 91(3-5), 139-149. doi:10.1016/s0928-4257(97)89478-8
[8] Siemerink, M. J., Klaassen, I., Vogels, I. M., Griffioen, A. W., Van Noorden, C. J., & Schlingemann, R. O. (2012). CD34 marks angiogenic tip cells in human vascular endothelial cell cultures. Angiogenesis, 15(1), 151–163. https://doi.org/10.1007/s10456-011-9251-z
[9] Carabotti, M., Scirocco, A., Maselli, M. A., & Severi, C. (2015). The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Annals of gastroenterology, 28(2), 203–209.
[10] Rhee, S. H., Pothoulakis, C., & Mayer, E. A. (2009). Principles and clinical implications of the brain-gut-enteric microbiota axis. Nature reviews. Gastroenterology & hepatology, 6(5), 306–314. https://doi.org/10.1038/nrgastro.2009.35
[11] Sikiric, P., Seiwerth, S., Rucman, R., Kolenc, D., Vuletic, L. B., Drmic, D., Grgic, T., Strbe, S., Zukanovic, G., Crvenkovic, D., Madzarac, G., Rukavina, I., Sucic, M., Baric, M., Starcevic, N., Krstonijevic, Z., Bencic, M. L., Filipcic, I., Rokotov, D. S., & Vlainic, J. (2016). Brain-gut Axis and Pentadecapeptide BPC 157: Theoretical and Practical Implications. Current neuropharmacology, 14(8), 857–865. https://doi.org/10.2174/1570159x13666160502153022
[12] X M Cao, R A Koski, A Gashler, M McKiernan, C F Morris, R Gaffney, R V Hay, V P Sukhatme Molecular and Cellular Biology May 1990, 10 (5) 1931-1939; DOI: 10.1128/MCB.10.5.1931
[13] González-Aguilera, C., Tous, C., Babiano, R., de la Cruz, J., Luna, R., & Aguilera, A. (2011). Nab2 functions in the metabolism of RNA driven by polymerases II and III. Molecular biology of the cell, 22(15), 2729–2740. https://doi.org/10.1091/mbc.E11-01-0055
[14] Sikiric, P., Seiwerth, S., Rucman, R., Turkovic, B., Rokotov, D. S., Brcic, L., . . . Sebecic, B. (2012). Focus on Ulcerative Colitis: Stable Gastric Pentadecapeptide BPC 157. Current Medicinal Chemistry, 19(1), 126-132. doi:10.2174/092986712803414015
[15] Seiwerth, S., Rucman, R., Turkovic, B., Sever, M., Klicek, R., Radic, B., Drmic, D., Stupnisek, M., Misic, M., Vuletic, L. B., Pavlov, K. H., Barisic, I., Kokot, A., Japjec, M., Blagaic, A. B., Tvrdeic, A., Rokotov, D. S., Vrcic, H., Staresinic, M., … Sikiric, P. (2018). BPC 157 and Standard Angiogenic Growth Factors. Gastrointestinal Tract Healing, Lessons from Tendon, Ligament, Muscle and Bone Healing. Current Pharmaceutical Design, 24(18), 1972–1989. https://doi.org/10.2174/1381612824666180712110447
[16] Tarnawski, A. S., Ahluwalia, A., & Jones, M. K. (2014). Increased susceptibility of aging gastric mucosa to injury: the mechanisms and clinical implications. World journal of gastroenterology, 20(16), 4467–4482. https://doi.org/10.3748/wjg.v20.i16.4467
[17] Love S. (2006). Demyelinating diseases. Journal of clinical pathology, 59(11), 1151–1159. https://doi.org/10.1136/jcp.2005.031195
[18] Neubauer H, Cumano A, Müller M, Wu H, Huffstadt U, Pfeffer K (May 1998). "Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis". Cell. 93 (3): 397–409. doi:10.1016/S0092-8674(00)81168-X. PMID 9590174.
[19] Sikiric, P., Jelovac, N., Jelovac-Gjeldum, A., Dodig, G., Staresinic, M., Anic, T., Zoricic, I., Rak, D., Perovic, D., Aralica, G., Buljat, G., Prkacin, I., Lovric-Bencic, M., Separovic, J., Seiwerth, S., Rucman, R., Petek, M., Turkovic, B., Ziger, T., Boban-Blagaic, A., … Babic, S. (2002). Pentadecapeptide BPC 157 attenuates chronic amphetamine-induced behavior disturbances. Acta pharmacologica Sinica, 23(5), 412–422.
[20] Boban Blagaic, A., Blagaic, V., Mirt, M., Jelovac, N., Dodig, G., Rucman, R., Petek, M., Turkovic, B., Anic, T., Dubovecak, M., Staresinic, M., Seiwerth, S., & Sikiric, P. (2005). Gastric pentadecapeptide BPC 157 effective against serotonin syndrome in rats. European journal of pharmacology, 512(2-3), 173–179. https://doi.org/10.1016/j.ejphar.2005.02.033
[21] Peters R. (2006). Ageing and the brain. Postgraduate medical journal, 82(964), 84–88. https://doi.org/10.1136/pgmj.2005.036665
[22] O’Mahony et al., 2015 S.M. O’Mahony, G. Clarke, Y.E. Borre, T.G. Dinan, J.F. Cryan
Serotonin, tryptophan metabolism and the brain-gut-microbiome axis
Behav. Brain Res. (2015), 10.1016/j.bbr.2014.07.027
[23] National Center for Biotechnology Information (2020). PubChem Compound Summary for CID 108101. Retrieved August 4, 2020 from https://pubchem.ncbi.nlm.nih.gov/compound/108101.
[24] Sian J, Youdim MBH, Riederer P, et al. MPTP-Induced Parkinsonian Syndrome. In: Siegel GJ, Agranoff BW, Albers RW, et al., editors. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th edition. Philadelphia: Lippincott-Raven; 1999. Available from: https://www.ncbi.nlm.nih.gov/books/NBK27974/
[25] Sikiric P., Marovic A., Matoz W., Anic T., Buljat G., Mikus D., Stancic-Rokotov D., Separovic J., Seiwerth S., Grabarevic Z., Rucman R., Petek M., Ziger T., Sebecic B., Zoricic I., Turkovic B., Aralica G., Perovic D., Duplancic B., Lovric-Bencic M., Rotkvic I., Mise S., Jagic V., Hahn V. A behavioral study of the effect of pentadecapeptide BPC 157 in Parkinson’s disease models in mice and gastric lesions induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydrophyridine. J. Physiol. Paris. 1999;93(6):505–512. [http://dx.doi.org/10.1016/S0928-4257(99) 00119-9]. [PMID: 10672997].
[26] Sikiric, P., Seiwerth, S., Rucman, R., Turkovic, B., Stancic Rokotov, D., Brcic, L., Sever, M., Klicek, R., Radic, B., Drmic, D., Ilic, S., Kolenc, D., Vrcic, H., & Sebecic, B. (2011). Stable gastric Pentadecapeptide BPC 157: Novel therapy in gastrointestinal tract. Current
Pharmaceutical Design, 17(16), 1612-1632. https://doi.org/10.2174/138161211796196954
[27] Pentadecapeptide BPC 157 interaction with other systems in gastric protection. (1995). Gastroenterology, 108(4), A220. https://doi.org/10.1016/0016-5085(95)23539-6
[28] Huang, T., Zhang, K., Sun, L., Xue, X., Zhang, C., Shu, Z., Mu, N., Gu, J., Zhang, W., Wang, Y., Zhang, Y., & Zhang, W. (2015). Body protective compound-157 enhances alkali-burn wound healing in vivo and promotes proliferation, migration, and angiogenesis in vitro. Drug design, development and therapy, 9, 2485–2499. https://doi.org/10.2147/DDDT.S82030
[29] Filosevic, A., Waldowski, R. A., Vidovic, T., Sikiric, P., & Drmic, D. (2017). Stable gastric pentadecapeptide BPC 157 antagonizes hydrogen peroxide induced oxidative stress in Drosophila melanogaster. The FASEB Journal, 31(1_supplement), 667-14.[30] Chang, C. H., Tsai, W. C., Lin, M. S., Hsu, Y. H., & Pang, J. H. S. (2011). The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. Journal of applied physiology.
[31] Park, J. M., Lee, H. J., Sikiric, P., & Hahm, K. B. (2020). BPC157 Rescued NSAID-cytotoxicity Via Stabilizing Intestinal Permeability and Enhancing Cytoprotection. Current Pharmaceutical Design.
[32] Sikirić, P., Petek, M., Ručman, R., Seiwerth, S., Grabarević, Z., Rotkvić, I., ... & Lang, N. (1993). A new gastric juice peptide, BPC. An overview of the stomach-stress-organoprotection hypothesis and beneficial effects of BPC. Journal of Physiology-Paris, 87(5), 313-327.
[33] Seiwerth, S., Brcic, L., Batelja Vuletic, L., Kolenc, D., Aralica, G., Misic, M., ... & Sikiric, P. (2014). BPC 157 and blood vessels. Current pharmaceutical design, 20(7), 1121-1125.
[34] Seiwerth, S., Sikiric, P., Grabarevic, Z., Zoricic, I., Hanzevacki, M., Ljubanovic, D., ... & Turkovic, B. (1997). BPC 157's effect on healing. Journal of Physiology-Paris, 91(3-5), 173-178.
[35] Xu, K., Sun, X., Puchowicz, M. A., & LaManna, J. C. (2008). Increased sensitivity to transient global ischemia in aging rat brain. In Oxygen Transport to Tissue XXVIII (pp. 199-206). Springer, Boston, MA.
[36] Davis, M., Mendelow, A. D., Perry, R. H., Chambers, I. R., & James, O. F. W. (1995). Experimental stroke and neuroprotection in the aging rat brain. Stroke, 26(6), 1072-1078.
[37] Ware, J. A., & Simons, M. (1997). Angiogenesis in ischemic heart disease. Nature medicine, 3(2), 158-164.
[38] Tomita, S., Mickle, D. A., Weisel, R. D., Jia, Z. Q., Tumiati, L. C., Allidina, Y., ... & Li, R. K. (2002). Improved heart function with myogenesis and angiogenesis after autologous porcine bone marrow stromal cell transplantation. The Journal of thoracic and cardiovascular surgery, 123(6), 1132-1140.
[39] Bosch-Marce, M., Okuyama, H., Wesley, J. B., Sarkar, K., Kimura, H., Liu, Y. V., ... & Zhou, Y. F. (2007). Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circulation research, 101(12), 1310-1318.
[40] Koike, T., Vernon, R. B., Gooden, M. D., Sadoun, E., & Reed, M. J. (2003). Inhibited angiogenesis in aging: a role for TIMP-2. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 58(9), B798-B805.
[41] Sadoun, E., & Reed, M. J. (2003). Impaired angiogenesis in aging is associated with alterations in vessel density, matrix composition, inflammatory response, and growth factor expression. Journal of Histochemistry & Cytochemistry, 51(9), 1119-1130.
[42] Mechanisms of adaptive angiogenesis to tissue hypoxia.
Fong GH
Angiogenesis. 2008; 11(2):121-40.
[43] Pugh C, Ratcliffe P. Regulation of angiogenesis by hypoxia: Role of the hif system. Nat Med. 2003;9:677–684.
[44] Lee S, Wolf P, Escudero R, Deutsch R, Jamieson S, Thistlethwaite P. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N Engl J Med. 2000;342:626–633.
[45] Hu, K., Babapoor-Farrokhran, S., Rodrigues, M., Deshpande, M., Puchner, B., Kashiwabuchi, F., Hassan, S. J., Asnaghi, L., Handa, J. T., Merbs, S., Eberhart, C. G., Semenza, G. L., Montaner, S., & Sodhi, A. (2016). Hypoxia-inducible factor 1 upregulation of both VEGF and ANGPTL4 is required to promote the angiogenic phenotype in uveal melanoma. Oncotarget, 7(7), 7816–7828. https://doi.org/10.18632/oncotarget.6868
[46] Zhang, K., Lu, J., Mori, T., Smith-Powell, L., Synold, T. W., Chen, S., & Wen, W. (2011). Baicalin increases VEGF expression and angiogenesis by activating the ERR{alpha}/PGC-1{alpha} pathway. Cardiovascular research, 89(2), 426–435. https://doi.org/10.1093/cvr/cvq296
[47] Yeo E. J. (2019). Hypoxia and aging. Experimental & molecular medicine, 51(6), 1–15. https://doi.org/10.1038/s12276-019-0233-3
[48] Lubomirov, L. T., Papadopoulos, S., Pütz, S., Welter, J., Klöckener, T., Weckmüller, K., Ardestani, M. A., Filipova, D., Metzler, D., Metzner, H., Staszewski, J., Zittrich, S., Gagov, H., Schroeter, M. M., & Pfitzer, G. (2017). Aging-related alterations in eNOS and nNOS responsiveness and smooth muscle reactivity of murine basilar arteries are modulated by apocynin and phosphorylation of myosin phosphatase targeting subunit-1. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism, 37(3), 1014–1029. https://doi.org/10.1177/0271678X16649402
[49] Chen, K., Pittman, R. N., & Popel, A. S. (2008). Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective. Antioxidants & redox signaling, 10(7), 1185–1198. https://doi.org/10.1089/ars.2007.1959
[50] Hsieh, M. J., Liu, H. T., Wang, C. N., Huang, H. Y., Lin, Y., Ko, Y. S., Wang, J. S., Chang, V. H., & Pang, J. S. (2017). Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation. Journal of molecular medicine (Berlin, Germany), 95(3), 323–333.
[51] Seiwerth, S., Sikiric, P., Grabarevic, Z., Zoricic, I., Hanzevacki, M., Ljubanovic, D., Coric, V., Konjevoda, P., Petek, M., Rucman, R., Turkovic, B., Perovic, D., Mikus, D., Jandrijevic, S., Medvidovic, M., Tadic, T., Romac, B., Kos, J., Peric, J., & Kolega, Z. (1997). BPC 157’s effect on healing. Journal of Physiology-Paris, 91(3–5), 173–178. https://doi.org/10.1016/s0928-4257(97)89480-6
[52] Hsieh, M.-J., Liu, H.-T., Wang, C.-N., Huang, H.-Y., Lin, Y., Ko, Y.-S., Wang, J.-S., Chang, V. H.-S., & Pang, J.-H. S. (2016). Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation. Journal of Molecular Medicine, 95(3), 323–333. https://doi.org/10.1007/s00109-016-1488-y
[53]Cerovecki, T., Bojanic, I., Brcic, L., Radic, B., Vukoja, I., Seiwerth, S., & Sikiric, P. (2010). Pentadecapeptide BPC 157 (PL 14736) improves ligament healing in the rat. Journal of orthopaedic research, 28(9), 1155-1161.
[54] Balenovic, D., Bencic, M. L., Udovicic, M., Simonji, K., Hanzevacki, J. S., Barisic, I., ... & Coric, M. (2009). Inhibition of methyldigoxin-induced arrhythmias by pentadecapeptide BPC 157: a relation with NO-system. Regulatory peptides, 156(1-3), 83-89.
[55] Pipili‐Synetos, E., Sakkoula, E., & Maragoudakis, M. E. (1993). Nitric oxide is involved in the regulation of angiogenesis. British journal of pharmacology, 108(4), 855-857.
[56] Cooke, J. P. (2003). NO and angiogenesis. Atherosclerosis Supplements, 4(4), 53-60.
[57] Alaiti, M. A., Ishikawa, M., Masuda, H., Simon, D. I., Jain, M. K., Asahara, T., & Costa, M. A. (2012). Up‐regulation of miR‐210 by vascular endothelial growth factor in ex vivo expanded CD 34+ cells enhances cell‐mediated angiogenesis. Journal of cellular and molecular medicine, 16(10), 2413-2421.
[58] Clara, C. A., Marie, S. K., de Almeida, J. R. W., Wakamatsu, A., Oba‐Shinjo, S. M., Uno, M., ... & Rosemberg, S. (2014). Angiogenesis and expression of PDGF‐C, VEGF, CD 105 and HIF‐1α in human glioblastoma. Neuropathology, 34(4), 343-352.
[59] Eming, S. A., Brachvogel, B., Odorisio, T., & Koch, M. (2007). Regulation of angiogenesis: wound healing as a model. Progress in histochemistry and cytochemistry, 42(3), 115-170.
[60] Pettet, G. J., Byrne, H. M., McElwain, D. L. S., & Norbury, J. (1996). A model of wound-healing angiogenesis in soft tissue. Mathematical biosciences, 136(1), 35-63.
[61] Gwyer, D., Wragg, N. M., & Wilson, S. L. (2019). Gastric pentadecapeptide body protection compound BPC 157 and its role in accelerating musculoskeletal soft tissue healing. Cell and tissue research, 1-7.
[62] Peake, J., Gatta, P. D., & Cameron-Smith, D. (2010). Aging and its effects on inflammation in skeletal muscle at rest and following exercise-induced muscle injury. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 298(6), R1485-R1495.
[63] Hsieh, M.-J., Liu, H.-T., Wang, C.-N., Huang, H.-Y., Lin, Y., Ko, Y.-S., Wang, J.-S., Chang, V. H.-S., & Pang, J.-H. S. (2016). Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation. Journal of Molecular Medicine, 95(3), 323–333. https://doi.org/10.1007/s00109-016-1488-y
[64] Staresinic, M., Petrovic, I., Novinscak, T., Jukic, I., Pevec, D., Suknaic, S., ... & Zoric, Z. (2006). Effective therapy of transected quadriceps muscle in rat: gastric pentadecapeptide BPC 157. Journal of orthopaedic research, 24(5), 1109-1117.
[65] Foster, J. A., & Neufeld, K. A. M. (2013). Gut–brain axis: how the microbiome influences anxiety and depression. Trends in neurosciences, 36(5), 305-312.
[66] Cryan, J. F., & O’mahony, S. M. (2011). The microbiome‐gut‐brain axis: from bowel to behavior. Neurogastroenterology & Motility, 23(3), 187-192.
[67] Mayer, E. A., Tillisch, K., & Gupta, A. (2015). Gut/brain axis and the microbiota. The Journal of clinical investigation, 125(3), 926-938.
[68] Bonaz, B., Bazin, T., & Pellissier, S. (2018). The vagus nerve at the interface of the microbiota-gut-brain axis. Frontiers in neuroscience, 12, 49.
[69] Xue, X. C., Wu, Y. J., Gao, M. T., Li, W. G., Zhao, N., Wang, Z. L., ... & Zhang, Y. Q. (2004). Protective effects of pentadecapeptide BPC 157 on gastric ulcer in rats. World journal of gastroenterology, 10(7), 1032.
[70] Ilić, S., Brčić, I., Mešter, M., Filipović, M., Sever, M., Kliček, R., ... & Berkopić, L. (2009). Over-dose insulin and stable gastric pentadecapeptide BPC 157. attenuated gastric ulcers, seizures, brain lesions, hepatomegaly, fatty liver, breakdown of liver glycogen, profound hypoglycemia and calcification in rats. Journal of physiology and pharmacology, 60(S7), 107.
[71] Jelovac, N., Sikiric, P., Rucman, R., Petek, M., Marovic, A., Perovic, D., ... & Miklic, P. (1999). Pentadecapeptide BPC 157 attenuates disturbances induced by neuroleptics: the effect on catalepsy and gastric ulcers in mice and rats. European journal of pharmacology, 379(1), 19-31.
[72] Tkalcević, V. I., Cuzić, S., Brajsa, K., Mildner, B., Bokulić, A., Situm, K., Perović, D., Glojnarić, I., & Parnham, M. J. (2007). Enhancement by PL 14736 of granulation and collagen organization in healing wounds and the potential role of egr-1 expression. European journal of pharmacology, 570(1-3), 212–221. https://doi.org/10.1016/j.ejphar.2007.05.072
[73] Chang, C. H., Tsai, W. C., Lin, M. S., Hsu, Y. H., & Pang, J. H. (2011). The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. Journal of applied physiology (Bethesda, Md. : 1985), 110(3), 774–780. https://doi.org/10.1152/japplphysiol.00945.2010
[74] .-H. Chang, W.-C. Tsai, Y.-H. Hsu, and J.-H. Pang, “Pen-tadecapeptide BPC 157 enhances the growth hormone re-ceptor expression in tendon fibroblasts,”Molecules, vol. 19,no. 11, pp. 19066–19077, 2014.
[75] Topilko, P., Levi, G., Merlo, G., Mantero, S., Desmarquet, C., Mancardi, G., & Charnay, P. (1997). Differential regulation of the zinc finger genes Krox‐20 and Krox‐24 (Egr‐1) suggests antagonistic roles in Schwann cells. Journal of neuroscience research, 50(5), 702-712.
[76] Jones, E. A., Jang, S. W., Mager, G. M., Chang, L. W., Srinivasan, R., Gokey, N. G., Ward, R. M., Nagarajan, R., & Svaren, J. (2007). Interactions of Sox10 and Egr2 in myelin gene regulation. Neuron glia biology, 3(4), 377–387. https://doi.org/10.1017/S1740925X08000173
[77] St-Sauveur, V. G., Soucek, S., Corbett, A. H., & Bachand, F. (2013). Poly (A) tail-mediated gene regulation by opposing roles of Nab2 and Pab2 nuclear poly (A)-binding proteins in pre-mRNA decay. Molecular and cellular biology, 33(23), 4718-4731.
[78] Lucerna, M., Mechtcheriakova, D., Kadl, A., Schabbauer, G., Schäfer, R., Gruber, F., ... & Binder, B. R. (2003). NAB2, a corepressor of EGR-1, inhibits vascular endothelial growth factor-mediated gene induction and angiogenic responses of endothelial cells. Journal of Biological Chemistry, 278(13), 11433-11440.
[79] Sikiric, P., Hahm, K.-B., Blagaic, A. B., Tvrdeic, A., Pavlov, K. H., Petrovic, A., Kokot, A., Gojkovic, S., Krezic, I., Drmic, D., Rucman, R., & Seiwerth, S. (2020). Stable Gastric Pentadecapeptide BPC 157, Robert’s Stomach Cytoprotection/Adaptive Cytoprotection/Organoprotection, and Selye’s Stress Coping Response: Progress, Achievements, and the Future. Gut and Liver, 14(2), 153–167. https://doi.org/10.5009/gnl18490
[80] Tohyama, Y., Sikirić, P., & Diksic, M. (2004). Effects of pentadecapeptide BPC157 on regional serotonin synthesis in the rat brain: alpha-methyl-L-tryptophan autoradiographic measurements. Life sciences, 76(3), 345–357. https://doi.org/10.1016/j.lfs.2004.08.010
[81] Austin, J. H., Connole, E., Kett, D., & Collins, J. (1978). Studies in aging of the brain. V. Reduced norepinephrine, dopamine, and cyclic AMP in rat brain with advancing age. Age, 1(4), 121-124.
[82] Creasey, H., & Rapoport, S. I. (1985). The aging human brain. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 17(1), 2-10.
[83] Rinne, J. O. (1987). Muscarinic and dopaminergic receptors in the aging human brain. Brain Research, 404(1-2), 162-168.
[84] Rinne, J. O. (1987). Muscarinic and dopaminergic receptors in the aging human brain. Brain Research, 404(1-2), 162-168.
[85] de Keyser, J., De Backer, J. P., Vauquelin, G., & Ebinger, G. (1990). The effect of aging on the D1 dopamine receptors in human frontal cortex. Brain research, 528(2), 308-310.
[86] Wester, P., Hardy, J. A., Marcusson, J., Nyberg, P., & Winblad, B. (1984). Serotonin concentrations in normal aging human brains: relation to serotonin receptors. Neurobiology of aging, 5(3), 199-203.
[87] Kojic, L., Gu, Q., Douglas, R. M., & Cynader, M. S. (1997). Serotonin facilitates synaptic plasticity in kitten visual cortex: an in vitro study. Developmental Brain Research, 101(1-2), 299-304.
[88] Fernandez, S. P., Muzerelle, A., Scotto-Lomassese, S., Barik, J., Gruart, A., Delgado-García, J. M., & Gaspar, P. (2017). Constitutive and acquired serotonin deficiency alters memory and hippocampal synaptic plasticity. Neuropsychopharmacology, 42(2), 512-523.