Thymic Rejuvenation
Written & Presented by Dhivyan Kurani
Peer-reviewers: Aakash Hans & Madison King
Introduction:
Aging in humans is marked by the onset of deadly diseases such as heart diseases and cancer due to immunosenescence or a declined immune system function in the older population (Haroun, 2018). The involution of the thymus is partly responsible for this since, after puberty, the thymus begins to shrink and go through fatty and fibrotic changes. This results in the thymus losing its structural integrity, reduced thymocyte numbers, and decreased T lymphopoiesis (Dixit, 2010; Crooke et al., 2019). Therefore the emerging concept of thymic rejuvenation seeks to restore immune function as well as thymus tissue in the elderly in order to combat immunosenescence.
Aging is an inevitable part of human development that involves the deterioration and dysfunction of multiple organs within the human body and the onset of many diseases (Haroun, 2018). Reactive oxygen species (ROS) are one of the main contributing factors to aging and their levels in the body increase over the course of the human life cycle (Davalli et al., 2016). Reactive oxygen species are essentially highly reactive, free radicals that result from the reduction of molecular oxygen to superoxide (Hayyan et al., 2016). These are harmful to human health as they have the ability to cause degenerative diseases such as Parkinson’s disease as well as cataracts, chronic inflammation, heart diseases, and cancers due to their ability to damage DNA (Richter, 1992; Tavassolifar et al., 2020; Yang et al., 2013).
The thymus or thymus gland is a vital lymphoid organ located in the chest between the lungs and breastbone, used to maintain homeostasis within the peripheral immune system (Pearse, 2006; Ventevogel & Sempowski, 2014). It does this beginning with white blood cells being created in the bone marrow and then maturing in the thymus (Abbott, 2019). Within this organ, T cells are then produced in order to create a stronger and functional immune system through preventing autoimmune diseases, eliminating dangerous foreign elements, and fighting infections (Andersen et al., 2006; Vignali et al., 2008). However, during puberty, the thymus starts to shrink and then begins to go through fatty and fibrotic changes with increasing age. In fact, thymic aging, marked by a loss in the structural integrity of the thymus, reduced thymocyte numbers, decreased T lymphopoiesis, and disturbances in the microenvironment of the thymic stromal cell are faster in comparison to other organs within the human body. Ultimately this results in the thymus’ conversion into adipose tissue and subsequent immunosenescence or weakened immune system within the elderly population (Dixit, 2010; Crooke et al., 2019). In addition, as life expectancy increases, immunosenescence increases along with the rate of cancer and chronic diseases within this population. Hence, it is important to combat this challenge and keep the aging population healthy since their susceptibility to these life-debilitating diseases can put a strain on individuals as well as the overall healthcare system (Abusarah et al., 2018).
The involution of the thymus and the complications that arise from it could be combatedwith the recent and emerging idea of thymic rejuvenation, whereby the thymus is restored withinthe older demographic. As a result of this process, the immune response within these individualswill be stimulated and they will have a slower rate of immunosenescence. An improved immuneresponse can also increase the effectiveness of vaccinations within the elderly (Abusarah et al.,2018)
An early study in 1986 used a rat pituitary tumor cell line (GH3) and looked at how the integration of GH3 pituitary adenoma cells in 16 to 22-month-old female Wistar-Furth rats can reverse thymic aging (Yamashita et al., 1995). Within the study, the GH3 pituitary adenoma cells were used due to their ability to secrete prolactin and growth hormone and were implanted into the rats over the course of 2 months. In comparison to the control group that only had remnants of the thymus gland, it was found that the Wistar-Furth rats with the GH3 pituitary adenoma cells had a thymus gland. Moreover, it was found that the rats with the GH3 cells had a higher proliferation of T-cells from the splenocytes of the rats than the controls (Kelley et al., 1986). This T-cell rejuvenation in animals means a higher count of T cells such as blood naïve T cells that decrease in the elderly human population (Salam et al., 2013). This boost in T-cells could combat declined immunity in aging individuals and increase the ability for them to efficiently respond to allergens, tumors, and pathogens (Kumar et al., 2018).
One of the first human clinical trials involving thymic rejuvenation, called the Thymus Regeneration, Immunorestoration, and Insulin Mitigation (TRIIM) trial, was created based on the findings of the 1986 study on rats. It sought to reverse human aging by thymus rejuvenation and immunosenescent reversal in aging men. The participants within the clinical trial were 9 adult men ranging from 51 to 65 years old. A cocktail of drugs was then administered to these participants involving recombinant human growth hormone (rhGH) and 2 diabetic medications called metformin and dehydroepiandrosterone (DHEA) (Abbott, 2019; Fahy et al., 2019). The hormone rhGh was utilized based on knowledge from prior studies indicating that it had the ability to provide immune restoration in animals (Kelley et al., 1986) These diabetic medications were also utilized in the treatment to prevent interference within the thymic rejuvenation and immune restoration process due to growth hormone (GH) induced hyperinsulinemia, caused by a high circulating insulin level in blood in comparison to its regular level relative to blood glucose (Fahy et al., 2019; Thomas et al., 2019).
During the trial, the participants’ blood samples were taken, magnetic resonance imaging (MRI) was used, and their genomes were analyzed. Within the participant’s blood samples, the blood cell count was rejuvenated. During an analysis of participant MRI scans for thymus composition, it was found that the fat found in the thymus region was replaced with regenerated thymus tissue (Abbott, 2019). Finally, four different epigenetic clocks were used to measure epigenetic or biological age based on genomic DNA methylation levels: Horvath, Levine, Hannum, and GrimAge (Fahy et al., 2019; Fransquet et al., 2019). During this analysis, it was found that on average, the participants’ epigenetic age decreased 2.5 years after 12 months of the treatment (Fahy et al., 2019). Geneticist Steve Horvath at the University of California, Los Angeles who created the Horvath epigenetic clock and carried out the epigenetic analysis of the trial says that he “... expected to see slowing down of the clock, but not a reversal” and claimed that the results “... felt kind of futuristic” (Abbott, 2019).
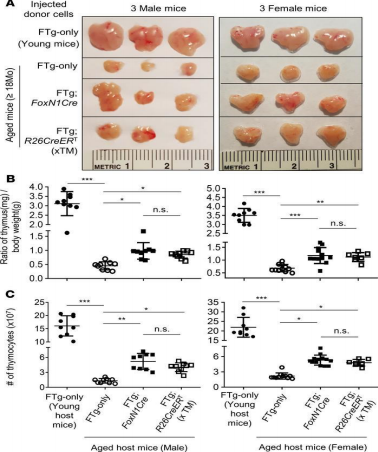
Another study took a different approach to thymic rejuvenation than the previous twostudies. This study was based on the knowledge that thymic involution is caused by thymicepithelial cells (TECs) having a lowered expression of the transcription factor forkhead box N1(FOXN1). The procedure used mice and surgically implanted Cre-mediatedFOXN1-reprogrammed embryonic fibroblasts (FREFs) into their aged thymus. After 45 days, ananalysis was then performed on the thymic mass of the mice in the study. It was found that themale and female mice treated with engrafted with the FREFs had a substantial amount of agethymus regrowth, along with rejuvenated function and architecture. Additionally, it was foundthat there was a reduction in autoreactive thymocytes as well as senescent T cells (Oh et al.,2020).
Future Research:
Additional studies involving human participants of various older ages and ethnicities in the Thymus Regeneration, Immunorestoration, and Insulin Mitigation (TRIIM) trial
The length at which the restoration of the thymus from the TRIIM trial lasts in humans
Implications for the future of healthcare in the older populations who utilize thymic rejuvenation therapies
Additional methods and approaches to restore thymic function in older populations
References
Abbott, A. (2019). First hint that body’s ‘biological age’ can be reversed. Nature, 573(7773). https://doi.org/10.1038/d41586-019-02638-w
Abusarah, J., Khodayarian, F., Cui, Y., El-Kadiry, A. E.-H., & Rafei, M. (2018). Thymic Rejuvenation: Are We There Yet? In Gerontology. InTech.
https://doi.org/10.5772/intechopen.74048
Andersen, M. H., Schrama, D., thor Straten, P., & Becker, J. C. (2006). Cytotoxic T Cells. Journal of Investigative Dermatology, 126(1). https://doi.org/10.1038/sj.jid.5700001
Crooke, S. N., Ovsyannikova, I. G., Poland, G. A., & Kennedy, R. B. (2019). Immunosenescence and human vaccine immune responses. Immunity & Ageing, 16(1).
https://doi.org/10.1186/s12979-019-0164-9
Davalli, P., Mitic, T., Caporali, A., Lauriola, A., & D’Arca, D. (2016). ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxidative Medicine and Cellular Longevity, 2016. https://doi.org/10.1155/2016/3565127
Dixit V. D. (2010). Thymic fatness and approaches to enhance thymopoietic fitness in aging. Current opinion in immunology, 22(4), 521–528. https://doi.org/10.1016/j.coi.2010.06.010
Fahy, G. M., Brooke, R. T., Watson, J. P., Good, Z., Vasanawala, S. S., Maecker, H., ... & Horvath, S. (2019). Reversal of epigenetic aging and immunosenescent trends in humans. Aging cell, 18(6), e13028.
Fransquet, P. D., Wrigglesworth, J., Woods, R. L., Ernst, M. E., & Ryan, J. (2019). The epigenetic clock as a predictor of disease and mortality risk: a systematic review and meta-analysis. Clinical Epigenetics, 11(1). https://doi.org/10.1186/s13148-019-0656-7
Fu, A. Z., Jiang, J. Z., Reeves, J. H., Fincham, J. E., Liu, G. G., & Perri III, M. (2007). Potentially inappropriate medication use and healthcare expenditures in the US community-dwelling elderly. Medical care, 472-476.
Haroun, H. S. (2018). Aging of thymus gland and immune system. MOJ Anatomy & Physiology, 5(2). https://doi.org/10.15406/mojap.2018.05.00186
Hayyan, M., Hashim, M. A., & AlNashef, I. M. (2016). Superoxide ion: generation and chemical implications. Chemical reviews, 116(5), 3029-3085.
Kelley, K. W., Brief, S., Westly, H. J., Novakofski, J., Bechtel, P. J., Simon, J., & Walker, E. B. (1986). GH3 pituitary adenoma cells can reverse thymic aging in rats. Proceedings of the National Academy of Sciences, 83(15). https://doi.org/10.1073/pnas.83.15.5663
Kumar, B. v., Connors, T. J., & Farber, D. L. (2018). Human T Cell Development, Localization, and Function throughout Life. Immunity, 48(2).
https://doi.org/10.1016/j.immuni.2018.01.007
Oh, J., Wang, W., Thomas, R., & Su, D. M. (2020). Thymic rejuvenation via FOXN1-reprogrammed embryonic fibroblasts (FREFs) to counteract age-related inflammation. JCI insight, 5(18).
Pearse, G. (2006). Normal Structure, Function and Histology of the Thymus. Toxicologic Pathology, 34(5). https://doi.org/10.1080/01926230600865549
Richter, C. (1992). Reactive oxygen and DNA damage in mitochondria. Mutation Research/DNAging, 275(3–6). https://doi.org/10.1016/0921-8734(92)90029-O
Salam N, Rane S, Das R, et al. T cell ageing: effects of age on development, survival & function. Indian J Med Res. 2013;138(5):595-608.
Singh, J., Mohtashami, M., Anderson, G., & Zúñiga-Pflücker, J. C. (2020). Thymic Engraftment by in vitro-Derived Progenitor T Cells in Young and Aged Mice. Frontiers in Immunology, 11. https://doi.org/10.3389/fimmu.2020.01850
Tavassolifar, M. javad, Vodjgani, M., Salehi, Z., & Izad, M. (2020). The Influence of Reactive Oxygen Species in the Immune System and Pathogenesis of Multiple Sclerosis. Autoimmune Diseases, 2020. https://doi.org/10.1155/2020/5793817
Thomas, D. D., Corkey, B. E., Istfan, N. W., & Apovian, C. M. (2019). Hyperinsulinemia: An Early Indicator of Metabolic Dysfunction. Journal of the Endocrine Society, 3(9). https://doi.org/10.1210/js.2019-00065 Ventevogel, M. S., & Sempowski, G. D. (2013). Thymic rejuvenation and aging. Current Opinion in Immunology, 25(4). https://doi.org/10.1016/j.coi.2013.06.002
Vignali, D. A. A., Collison, L. W., & Workman, C. J. (2008). How regulatory T cells work. Nature Reviews Immunology, 8(7). https://doi.org/10.1038/nri2343
Yamashita, H., Okadome, T., Franzén, P., ten Dijke, P., Heldin, C. H., & Miyazono, K. (1995). A rat pituitary tumor cell line (GH3) expresses type I and type II receptors and other cell surface binding protein(s) for transforming growth factor-beta. The Journal of biological chemistry, 270(2), 770–774. https://doi.org/10.1074/jbc.270.2.770
Yang, Y., Bazhin, A. v., Werner, J., & Karakhanova, S. (2013). Reactive Oxygen Species in the Immune System. International Reviews of Immunology, 32(3).