Spermidine, Autophagy and Longevity
Written by Dr. Aakash Hans
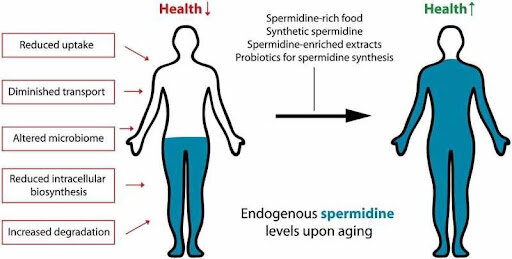
Spermidine is a naturally occurring polyamine known to promote the process of autophagy. Autophagy is an essential function which enables the body to rid itself of unwanted substances like improperly folded proteins, damaged cellular components and pathological microbes. There are three major types of autophagy : macro-autophagy, micro-autophagy and chaperone mediated autophagy (Glick et al., 2010). Spermidine is particularly involved with macro-autophagy. Supplementing animal models with spermidine has demonstrated lifespan-extending benefits. The levels of this polyamine decrease with age in humans and introducing foods rich in spermidine is believed to reduce death rates associated with cancer and heart disease (Madeo et al., 2019).
Antonie van Leeuwenhoek was responsible for discovering spermidine in the year of 1678, while its structure and functions were elicited in the first half of the 1900s (Wallace, 2009). In mammalian cells, putrescine and spermine give rise to spermidine and its levels in the cytoplasm are determined by how rapidly it is metabolised via acetylation and oxidation, how often it is excreted out of the cell and taken up by the extracellular space (Kee et al., 2004; Pegg, 2009). In humans intestinal bacteria are another source of spermidine and can be modulated by using probiotics to increase spermidine levels (Matsumoto & Benno, 2007). Spermidine obtained from diet is rapidly absorbed and distributed in the body (Milovic, 2001).
Foods with high spermidine content can be used to raise its levels, which are derived from plants or fermentation processes. Cauliflower, broccoli, wheat germ, mushrooms (shitake), green pepper and the fruit-durian are some of the plant based sources, while soybean foods and cheese are the fermentation based sources (Kiechl et al., 2018). Breast milk also contains spermidine and spermine as neonatal periods have higher requirements for these polyamines. Meats like beef, chicken and pork contain higher levels of spermine compared to spermidine. The intake of polyamines was estimated to be around 354 µmol/day in European adults, with higher levels recorded in Mediterranean areas and lower levels in the UK (Muñoz-Esparza et al., 2019). While there are no set recommendations for daily polyamine intake, (Ali et al., 2011) suggested 540 µmol/day which is higher than normal levels found by studies on humans.
Spermidine acts as an inhibitor of the enzyme EP300, which is an acetyl transferase enzyme. The enzyme is named after a protein coding gene - EP300, which stands for E1A binding protein P300. Many proteins are involved in the regulation of the autophagy process. These proteins have lysine residues, which if acetylated by enzymes like EP300, cause dysregulation of autophagic processes and hence leads to aging (F Pietrocola et al., 2015). Acetyl-coenzyme A (ACo-A) donates acetyl groups to acetylating enzymes (EP300) and enables them to inhibit autophagy. Therefore decreased levels of ACo-A lead to increased autophagy and vice versa as decreased levels of ACo-A correlate with reduced activity of acetylating enzymes (Mariño et al., 2014). The main action of spermidine is competing with EP300 and other enzymes for acetyl groups donated by ACo-A. Therefore, spermidine diminishes the capacity of EP300 to suppress autophagy and acts as autophagy inducer (F Pietrocola et al., 2015). This autophagy inducing effect of spermidine is comparable and equivalent to rapamycin which although is an immunosuppressant drug, also has autophagy stimulating properties (Madeo et al., 2019). The connection between spermidine and autophagy is bolstered by a study demonstrating that models in which genetic mutations affected autophagy showed no beneficial effects of spermidine on longevity (Eisenberg et al., 2009).
Since spermidine levels decline with age, a diet rich in polyamines can be used to encourage healthy aging. Studies in mice and humans showed a statistically significant increase in spermine levels after introducing a diet containing 50 to 100 grams daily of natto, a fermented soybean product (Soda et al., 2009). The safety of spermidine in mice and human subjects was demonstrated by a 3 month long study using wheat germ. No significant side effects were noted in the subjects given wheat germ compared to the placebo group (Schwarz et al., 2018). These findings will allow for studies of longer durations to better understand the effects of spermidine on cognition and brain health.
Two studies were reported in a single paper, both of which analysed the effects of dietary spermidine on human health. A group of 829 healthy subjects were followed up for a period of 15 years and their dietary intake was noted and analysed through questionnaires (Kiechl et al., 2018). Subjects who had a higher intake of spermidine containing foods showed lower rates of cancer and heart disease. This effect remained statistically significant after several potential confounding factors were corrected and the data was re-analysed. This was the first study to elucidate benefits of dietary spermidine.
A limitation of the mentioned studies is lack of lab values, especially ones correlating serum spermidine levels to the amount of autophagy taking place in the body. A potential way to achieve this could be to quantitatively measure levels of acetylated proteins and flux of autophagy through the study of mononuclear cells in the blood. Similar concept was used to study the link between fasting and autophagy (Federico Pietrocola et al., 2017). The dysregulation of polyamines causes havoc in the body as uncontrolled production and levels are seen in cancer and Alzheimer’s patients (Lipton et al., 1975; Pan et al., 2016). Hence another use of polyamines given exogenously could be to decrease raised levels of endogenous polyamines in cancer patients via a possible feedback mechanism (Madeo, Eisenberg, et al., 2018).
Spermidine is known to mimic the effects of calorie restriction as both induce and encourage autophagic processes in the body (Madeo et al., 2014). Additionally, spermidine and aspirin have similar targets they act on i.e. EP300 (Federico Pietrocola et al., 2018). Knowing the tremendous benefits aspirin has provided in terms of heart disease and mortality, future studies may conclude similar findings for spermidine. A safe approach to gaining the benefits of spermidine and other polyamines would be to develop a polyamine-rich diet and use probiotics to help the gut bacteria synthesize more spermidine (Madeo, Carmona-Gutierrez, et al., 2018).
References
Ali, M. A., Poortvliet, E., Strömberg, R., & Yngve, A. (2011). Polyamines: total daily intake in adolescents compared to the intake estimated from the Swedish Nutrition Recommendations Objectified (SNO). Food & Nutrition Research, 55. https://doi.org/10.3402/fnr.v55i0.5455
Eisenberg, T., Knauer, H., Schauer, A., Büttner, S., Ruckenstuhl, C., Carmona-Gutierrez, D., Ring, J., Schroeder, S., Magnes, C., Antonacci, L., Fussi, H., Deszcz, L., Hartl, R., Schraml, E., Criollo, A., Megalou, E., Weiskopf, D., Laun, P., Heeren, G., … Madeo, F. (2009). Induction of autophagy by spermidine promotes longevity. Nature Cell Biology, 11(11), 1305–1314. https://doi.org/10.1038/ncb1975
Glick, D., Barth, S., & Macleod, K. F. (2010). Autophagy: cellular and molecular mechanisms. The Journal of Pathology, 221(1), 3–12. https://doi.org/10.1002/path.2697
Kee, K., Foster, B. A., Merali, S., Kramer, D. L., Hensen, M. L., Diegelman, P., Kisiel, N., Vujcic, S., Mazurchuk, R. V, & Porter, C. W. (2004). Activated polyamine catabolism depletes acetyl-CoA pools and suppresses prostate tumor growth in TRAMP mice. The Journal of Biological Chemistry, 279(38), 40076–40083. https://doi.org/10.1074/jbc.M406002200
Kiechl, S., Pechlaner, R., Willeit, P., Notdurfter, M., Paulweber, B., Willeit, K., Werner, P., Ruckenstuhl, C., Iglseder, B., Weger, S., Mairhofer, B., Gartner, M., Kedenko, L., Chmelikova, M., Stekovic, S., Stuppner, H., Oberhollenzer, F., Kroemer, G., Mayr, M., … Willeit, J. (2018). Higher spermidine intake is linked to lower mortality: a prospective population-based study. The American Journal of Clinical Nutrition, 108(2), 371–380. https://doi.org/10.1093/ajcn/nqy102
Lipton, A., Sheehan, L. M., & Kessler, G. F. J. (1975). Urinary polyamine levels in human cancer. Cancer, 35(2), 464–468. https://doi.org/10.1002/1097-0142(197502)35:2<464::aid-cncr2820350225>3.0.co;2-8
Madeo, F., Bauer, M. A., Carmona-Gutierrez, D., & Kroemer, G. (2019). Spermidine: a physiological autophagy inducer acting as an anti-aging vitamin in humans? Autophagy, 15(1), 165–168. https://doi.org/10.1080/15548627.2018.1530929
Madeo, F., Carmona-Gutierrez, D., Kepp, O., & Kroemer, G. (2018). Spermidine delays aging in humans. Aging, 10(8), 2209–2211. https://doi.org/10.18632/aging.101517
Madeo, F., Eisenberg, T., Pietrocola, F., & Kroemer, G. (2018). Spermidine in health and disease. Science, 359(6374). https://doi.org/10.1126/science.aan2788
Madeo, F., Pietrocola, F., Eisenberg, T., & Kroemer, G. (2014). Caloric restriction mimetics: towards a molecular definition. In Nature reviews. Drug discovery (Vol. 13, Issue 10, pp. 727–740). https://doi.org/10.1038/nrd4391
Mariño, G., Pietrocola, F., Eisenberg, T., Kong, Y., Malik, S. A., Andryushkova, A., Schroeder, S., Pendl, T., Harger, A., Niso-Santano, M., Zamzami, N., Scoazec, M., Durand, S., Enot, D. P., Fernández, Á. F., Martins, I., Kepp, O., Senovilla, L., Bauvy, C., … Kroemer, G. (2014). Regulation of autophagy by cytosolic acetyl-coenzyme A. Molecular Cell, 53(5), 710–725. https://doi.org/10.1016/j.molcel.2014.01.016
Matsumoto, M., & Benno, Y. (2007). The relationship between microbiota and polyamine concentration in the human intestine: a pilot study. Microbiology and Immunology, 51(1), 25–35. https://doi.org/10.1111/j.1348-0421.2007.tb03887.x
Milovic, V. (2001). Polyamines in the gut lumen: bioavailability and biodistribution. European Journal of Gastroenterology & Hepatology, 13(9), 1021–1025. https://doi.org/10.1097/00042737-200109000-00004
Muñoz-Esparza, N. C., Latorre-Moratalla, M. L., Comas-Basté, O., Toro-Funes, N., Veciana-Nogués, M. T., & Vidal-Carou, M. C. (2019). Polyamines in Food. Frontiers in Nutrition, 6, 108. https://doi.org/10.3389/fnut.2019.00108
Pan, X., Nasaruddin, M. Bin, Elliott, C. T., McGuinness, B., Passmore, A. P., Kehoe, P. G., Hölscher, C., McClean, P. L., Graham, S. F., & Green, B. D. (2016). Alzheimer’s disease-like pathology has transient effects on the brain and blood metabolome. Neurobiology of Aging, 38, 151–163. https://doi.org/10.1016/j.neurobiolaging.2015.11.014
Pegg, A. E. (2009). Mammalian polyamine metabolism and function. IUBMB Life, 61(9), 880–894. https://doi.org/10.1002/iub.230
Pietrocola, F, Lachkar, S., Enot, D. P., Niso-Santano, M., Bravo-San Pedro, J. M., Sica, V., Izzo, V., Maiuri, M. C., Madeo, F., Mariño, G., & Kroemer, G. (2015). Spermidine induces autophagy by inhibiting the acetyltransferase EP300. Cell Death and Differentiation, 22(3), 509–516. https://doi.org/10.1038/cdd.2014.215
Pietrocola, Federico, Castoldi, F., Markaki, M., Lachkar, S., Chen, G., Enot, D. P., Durand, S., Bossut, N., Tong, M., Malik, S. A., Loos, F., Dupont, N., Mariño, G., Abdelkader, N., Madeo, F., Maiuri, M. C., Kroemer, R., Codogno, P., Sadoshima, J., … Kroemer, G. (2018). Aspirin Recapitulates Features of Caloric Restriction. Cell Reports, 22(9), 2395–2407. https://doi.org/10.1016/j.celrep.2018.02.024
Pietrocola, Federico, Demont, Y., Castoldi, F., Enot, D., Durand, S., Semeraro, M., Baracco, E. E., Pol, J., Bravo-San Pedro, J. M., Bordenave, C., Levesque, S., Humeau, J., Chery, A., Métivier, D., Madeo, F., Maiuri, M. C., & Kroemer, G. (2017). Metabolic effects of fasting on human and mouse blood in vivo. Autophagy, 13(3), 567–578. https://doi.org/10.1080/15548627.2016.1271513
Schwarz, C., Stekovic, S., Wirth, M., Benson, G., Royer, P., Sigrist, S. J., Pieber, T., Dammbrueck, C., Magnes, C., Eisenberg, T., Pendl, T., Bohlken, J., Köbe, T., Madeo, F., & Flöel, A. (2018). Safety and tolerability of spermidine supplementation in mice and older adults with subjective cognitive decline. Aging, 10(1), 19–33. https://doi.org/10.18632/aging.101354
Soda, K., Kano, Y., Sakuragi, M., Takao, K., Lefor, A., & Konishi, F. (2009). Long-term oral polyamine intake increases blood polyamine concentrations. Journal of Nutritional Science and Vitaminology, 55(4), 361–366. https://doi.org/10.3177/jnsv.55.361
Wallace, H. M. (2009). The polyamines: past, present and future. Essays in Biochemistry, 46, 1–9. https://doi.org/10.1042/bse0460001