NAD+: A Potential Treatment Option for COVID-19 Patients and Long Haulers
Written & Presented by Vanessa Zankich
Peer-reviewers: James Elwell & Amy Fabella
Introduction:
COVID-19 patients have exhibited overactive PARP-1 enzymes and dysregulated T-cells. These inappropriate immune responses have been linked to an exacerbation of symptom severity and higher prevalence of long COVID. Long COVID occurs when COVID-19 patients continue to suffer from symptoms long after the initial recovery period and end of the acute phase. This condition can lead to permanent systemic damage within the body so it is very important to determine effective ways in preventing long COVID from occurring in the first place. Experts have suggested that NAD+ can serve as a successful PARP-1 inhibitor and T-cell regulator, highlighting NAD+ as a perfect candidate for the treatment of COVID-19 and prevention of long COVID.
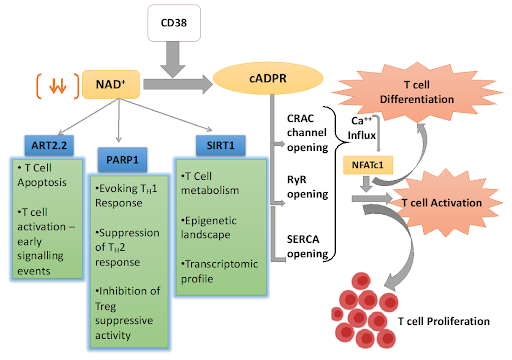
Before making any suggestions regarding potential treatments and therapies, it is important to first review what is known about the body’s immune response to an infection from SARS-CoV-2, the virus that causes COVID-19. It has been reported that COVID-19 patients exhibit a proinflammatory environment that is characterized by elevated levels of plasma cytokines and chemokines (Badawy, 2020). Past studies have shown that this elevation is higher and stronger in patients suffering from more severe infections, leading them to require intensive care (ICU) (Badawy). With that being said, this proinflammatory environment is observed in all COVID-19 patients regardless of symptom severity (Badawy). It is known that a proinflammatory environment can result in activation of immune modulating proteins aryl hydrocarbon receptor (AhR) and poly (ADP-ribose) polymerase 1 (PARP-1) (Badawy). PARP-1 is an enzyme that consumes NAD+; if NAD+ and adenosine triphosphate (ATP) are consumed at a higher rate than they can be replenished, as marked by PARP-1 hyperactivity or over-consumption, cell death can occur (Badawy). For this reason, it is possible that targeting and inhibiting the activity of PARP-1, thus preventing over-consumption of NAD+, may be a potential therapy of COVID-19.
Dysregulation of T-cells in COVID-19 patients
T-cells are central to an organism’s immune response, including those with an infection from the SARS-CoV-2 virus. Once T-cells are activated, some T-cells differentiate into effector T-cells, which produce cytokines and chemokines (Liu et al., 2020). Others differentiate into cytotoxic T-cells, which have the specific goal to kill virus-infected target cells (Liu et al.). T-cell responses in severely ill COVID-19 patients may be at inappropriate levels, whether that may be due to underactivation or overactivation (Rodriguez Cetina Biefer et al., 2017).
Clinical reports have shown that T-cell counts are lower in COVID-19 patients than in those without an active infection. In fact, 92.9% of those with moderate to severe SARS-CoV-2 infections had lower T-cell counts than the normal range (Liu et al.). A statistically significant difference in T-cell count between those with severe versus non-severe infections has been observed, indicating that T-cells play an imperative role in patient recovery (Liu et al.).
Reports have also shown that T-cell count increases in patients with SARS-CoV-2 infection, indicating T-cell activation and cytokine/chemokine production (Liu et al.). Some COVID-19 patients exhibit abnormally high levels of inflammatory cytokines, which indicates overactivation of T-cells. The higher the cytokine levels, the more severe the patient’s symptoms may be (Liu et al.). Overactivation of T-cells can also lead to cytokine storms, which occur when the body begins to attack its own cells rather than the target virus (Liu et al.). Such a reaction can lead to life-threatening systemic inflammation within the body. After reviewing this information, it becomes evident that COVID-19 therapy must address the dysregulation of T-cells within the body.
What does it mean to be a COVID-19 long hauler?
As we crawl into the second year of the COVID-19 pandemic, it has become apparent that some COVID-19 patients may continue to suffer through symptoms for weeks, and sometimes even months, past the initial recovery period and end of the acute phase (“Lingering Effects,” 2020). This occurrence observed is now clinically referred to as "long COVID." Those who endure long COVID are known as "long haulers” (“Lingering Effects”). Regardless of symptom severity, it has been observed that any patient suffering from the SARS-CoV-2 virus can enter into long hauler territory. However, it should be noted that patients with mild symptom severity who did not require hospitalization were less likely to become long haulers; the percentage of such an occurrence lands at around 10% (“Lingering Effects”). The fact that this number is relatively low should not lead one to believe that this is an insignificant incidence; recovering from long COVID can be extremely difficult and frightening for anyone, no matter how healthy one may be prior to a SARS-CoV-2 infection.
On the other hand, patients who did require hospitalization due to more severe symptoms were more likely to suffer from long COVID (“Lingering Effects”; Carfi et al., 2020; Garrigues et al., 2020). Italian researchers developed a study with the goal to assess persistent symptoms in patients who were hospitalized and recovered from COVID-19 (Carfi et al.). 143 patients were included in this study’s analysis; patients were assessed an average of 60.3 days (about 2 months) after onset of their first COVID-19 symptom. The researchers found that a hefty 87.4% of these discharged patients continued to suffer from COVID-19 symptoms at this checkpoint. Specifically, 32% experienced 1-2 symptoms and 55% had 3 or more symptoms. None were observed to be experiencing any signs or symptoms of acute or severe illness. A total of 44.1% reported a worsened quality of life. The most common symptoms were fatigue (53.1%), dyspnea (43.4%), joint pain (27.3%), and chest pain (21.7%) (Carfi et al.).
In another study that also looked at the continuation of symptoms and quality of life in discharged COVID-19 patients, 120 patients were assessed a mean of 110.9 days after hospital admission (Garrigues et al.). Comparisons were made between patients who did and did not require intensive care (ICU). No statistically significant difference was found between those who had ICU stays and those who did not in terms of post-acute symptom prevalence. A total of 55% of these patients reported fatigue, 42% reported dyspnoea, 34% reported loss of memory, 30.8% reported disordered sleeping, and 28% reported concentration disorders (Garrigues et al.).
Based on previous knowledge regarding other viral pulmonary infections, it is also a possibility that COVID long haulers will experience long-term pulmonary consequences (Salehi et al., 2020). Patients may experience significant impairments in pulmonary functioning in the first few months after recovering from the acute phase of the disease (Salehi et al.). Such damage to pulmonary functioning may become permanent, so it is vital to investigate potential preventative measures that can be taken to reduce the occurrence of long COVID. Patient age, comorbidities, history of smoking, length of hospital stay, symptom severity, and medications taken are all factors that may determine whether or not an individual becomes a long hauler (Salehi et al.). Considering the fact that one cannot directly manipulate patient age, comorbidities, history of smoking, length of hospital stay, or symptom severity, we must focus our attention on the only controllable factor listed: medications taken. Decreasing the severity of COVID symptoms through medication administration may prevent long COVID from occurring (Rodriguez Cetina Biefer et al.). It has been shown that PARP-1 overactivity and T-cell dysregulation are both exhibited by COVID-19 patients with severe infections (Badawy; Liu et al.). Therefore, medications that have been shown to counter these issues should be investigated further since decreasing symptom severity may be a potential way to prevent long COVID from developing.
Why does NAD+ qualify as a potential COVID-19 treatment?
NAD+ is a PARP-1 inhibitor
NAD+ is a natural coenzyme that is found in all living cells. Past studies have found that NAD+ regulates immune responses and has the ability to induce homeostasis in organisms (Rodriguez Cetina Biefer et al.). NAD+ has been shown to rewire metabolism, activate sirtuins (a family of signaling proteins involved in metabolic regulation), and maintain mitochondrial fitness (mitochondrial health is central to ensuring the energy production and survival of cells) (Rodriguez Cetina Biefer et al.). By introducing NAD+ back into the system, NAD+ levels rise and the PARP-1 enzyme is inhibited, thus preventing overconsumption of NAD+ and cell death (Rodriguez Cetina Biefer et al.).
2. NAD+ regulates T-cells
NAD+ also serves as a potent immune regulator (through tryptophan catabolism, a process which affects malignant tumor cell properties and anti-tumor immune responses) and has been shown to induce T-cell differentiation (Rodriguez Cetina Biefer et al.). It follows that NAD+ may lead to proper T-cell regulation in COVID-19 patients, boosting the proper immune response which can lead to a higher rate of recovery and prevention of long COVID.
After reviewing the literature available, it becomes evident that further research should be done to determine the effectiveness of NAD+ as a treatment/therapy of COVID-19 and as a prevention of long COVID.
Future Research:
Test NAD+ as a PARP-1 inhibitor and T-cell regulator in a clinical setting
Determine whether NAD+ can effectively lessen symptom severity in COVID-19 patients
Investigate NAD+ administration as an effective prevention of long COVID
Test different methods of NAD+ administration in order to determine which is the most effective in lessening symptom severity and preventing long COVID
Some potential administration methods that would be worth exploring: NAD+ oral tablets/infused supplements, NAD+ intravenous therapy, NAD+ injections
References
Badawy, A. (2020). Immunotherapy of COVID-19 with poly (ADP-ribose) polymerase inhibitors: starting with nicotinamide. Biosci Rep, 40(10). https://doi.org/10.1042/BSR20202856
Carfi A., et al. (2020). Persistent symptoms in patients after acute COVID-19. JAMA, 324(6). doi:10.1001/jama.2020.12603.
Garrigues, E., et al. (2020). Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. The Journal of Infection, 81(6). https://doi.org/10.1016/j.jinf.2020.08.029
Liu, L., et al. (2020). T cell response in patients with COVID-19. Blood Science, 2(3). doi: 10.1097/BS9.0000000000000050
The lingering effects of COVID-19. (2020). American Journal of Nursing, 120(12). https://doi.org/10.1097/01.NAJ.0000724176.20278.f5
Rodriguez Cetina Biefer, H., et al. (2017). Aspects of tryptophan and nicotinamide adenine dinucleotide in immunity: A new twist in an old tale. International Journal of Tryptophan Research. https://doi.org/10.1177/1178646917713491
Salehi, S., et al. (2020). Long-term pulmonary consequences of coronavirus disease 2019 (COVID-19). Journal of Thoracic Imaging, 35(4).
doi: 10.1097/RTI.0000000000000534